
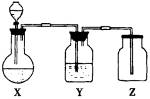
(1)ÖĘČ”Ä³ĪŽÉ«·Ē½šŹōŃõ»ÆĪļĘųĢåŹ±£¬ÉÕĘæXÄŚµÄŅ©Ę·Ó¦ŹĒ(””)
A£®ĶÓėĻ”HNO3””””””””””””””””B£®ĶÓėÅØHNO3
C£®CaCO3ÓėĻ”H2SO4””””””””””””D£®Na2SO3ÓėÅØHCl
(2)Ļ“ĘųĘæYÖŠĖł×°ŅŗĢåŹĒ(²»ŌŹŠķ·ĒĖ®ŌÓÖŹ»ģŌŚĘųĢåÖŠ)(””)
A£®ÅØH2SO4””””””””””””””””””””B£®NaOHČÜŅŗ
C£®±„ŗĶNaHSO3””””””””””””””””D£®NaHCO3ČÜŅŗ
(3)¼ģŃé¼ÆĘųĘæŹĒ·ń¼ÆĀśĘųĢ壬ÖĆÓŚĘææŚµÄĀĖÖ½Ó¦Õ“ÉĻµÄČÜŅŗŹĒ(””)
A£®BaCl2ČÜŅŗ”””””””””””””””””” B£®ĖįŠŌKMnO4ČÜŅŗ
C£®KIµķ·Ū””””””””””””””””””””””D£®ŹÆ»ŅĖ®
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĪļĄķ½ĢŃŠŹŅ ĢāŠĶ£ŗ022
£Ø1£©ÖĘČ”Ä³ĪŽÉ«·Ē½šŹōŃõ»ÆĪļĘųĢåŹ±£¬×¶ŠĪĘæX¼°·ÖŅŗĀ©¶·ÄŚµÄŅ©Ę·ŹĒ________”£
A£®ĶŗĶĻ”ĻõĖį”””””””””””””””””””””””””””””””””””””””””””””””””””” B£®ĶŗĶÅØĻõĖį
C£®Ģ¼ĖįøĘŗĶĻ”ĮņĖį”””””””””””””””””””””””””””””””””””””””””””” D£®ŃĒĮņĖįÄĘŗĶÅØŃĪĖį
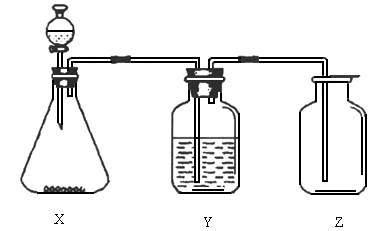
£Ø2£©Ļ“ĘųĘæYÖŠĖłŹ¢µÄŅŗĢåŹĒ________”£
A£®ÅØH2SO4”””””””””””””””””””””””””””””””””””””””””””””””””””””””” B£®ĒāŃõ»ÆÄĘČÜŅŗ
C£®ŃĒĮņĖįĒāÄĘČÜŅŗ”””””””””””””””””””””””””””””””””””””””””””” D£®Ģ¼ĖįĒāÄĘČÜŅŗ
£Ø3£©¼ģ²é¼ÆĘųĘæZŹĒ·ńŹÕ¼ÆĀśĘųĢ壬ÖĆÓŚĘææŚµÄĀĖÖ½Ó¦Õ“ÉĻµÄČÜŅŗŹĒ________”£
A£®ĀČ»Æ±µČÜŅŗ”””””””””””””””””””””””””””””””””””””””””””””””””””” B£®Ę·ŗģČÜŅŗ
C£®µā»Æ¼Ųµķ·ŪČÜŅŗ”””””””””””””””””””””””””””””””””””””””””””” D£®³ĪĒåŹÆ»ŅĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ022
ĻĀĮŠČżøöĪŹĢā¾łÓėČēĶ¼ĖłŹ¾ŹµŃé×°ÖĆÓŠ¹Ų£¬ÓĆŠņŗÅA”«D»Ų“š£ŗ
£Ø1£©ÖĘČ”Ä³ĪŽÉ«·Ē½šŹōŃõ»ÆĪļĘųĢåŹ±£¬×¶ŠĪĘæX¼°·ÖŅŗĀ©¶·ÄŚµÄŅ©Ę·ŹĒ________”£
A£®ĶŗĶĻ”ĻõĖį”””””””””””””””””””””””””””””””””””””””””””””””””””” B£®ĶŗĶÅØĻõĖį
C£®Ģ¼ĖįøĘŗĶĻ”ĮņĖį”””””””””””””””””””””””””””””””””””””””””””” D£®ŃĒĮņĖįÄĘŗĶÅØŃĪĖį
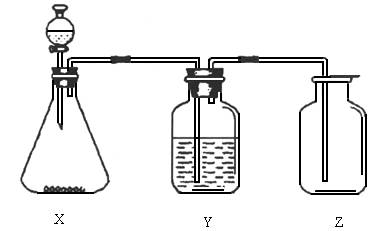
£Ø2£©Ļ“ĘųĘæYÖŠĖłŹ¢µÄŅŗĢåŹĒ________”£
A£®ÅØH2SO4”””””””””””””””””””””””””””””””””””””””””””””””””””””””” B£®ĒāŃõ»ÆÄĘČÜŅŗ
C£®ŃĒĮņĖįĒāÄĘČÜŅŗ”””””””””””””””””””””””””””””””””””””””””””” D£®Ģ¼ĖįĒāÄĘČÜŅŗ
£Ø3£©¼ģ²é¼ÆĘųĘæZŹĒ·ńŹÕ¼ÆĀśĘųĢ壬ÖĆÓŚĘææŚµÄĀĖÖ½Ó¦Õ“ÉĻµÄČÜŅŗŹĒ________”£
A£®ĀČ»Æ±µČÜŅŗ”””””””””””””””””””””””””””””””””””””””””””””””””””” B£®Ę·ŗģČÜŅŗ
C£®µā»Æ¼Ųµķ·ŪČÜŅŗ”””””””””””””””””””””””””””””””””””””””””””” D£®³ĪĒåŹÆ»ŅĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ»īĢāĒɽāĒÉĮ·”¤øßæ¼»Æѧ£ØµŚŅ»ĀÖ£© ĢāŠĶ£ŗ058
ĻĀĮŠČżøöĪŹĢā¾łÓėĶ¼ŹµŃé×°ÖĆÓŠ¹Ų£¬ÓĆŠņŗÅA”«D»Ų“š£ŗ
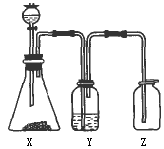
(1)ÖĘČ”Ä³ĪŽÉ«·Ē½šŹōŃõ»ÆĪļĘųĢåŹ±£¬×¶ŠĪĘæX¼°·ÖŅŗĀ©¶·ÄŚµÄŅ©Ę·ŹĒ
[””””]
(2)Ļ“ĘųĘæYÖŠĖłŹ¢µÄŅŗĢåŹĒ
[””””]
(3)¼ģ²é¼ÆĘųĘæZŹĒ·ńŹÕ¼ÆĀśĘųĢ壬ÖĆÓŚĘææŚµÄĀĖÖ½Ó¦Õ“ÉĻµÄČÜŅŗŹĒ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
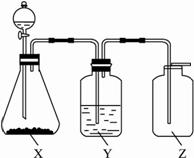 ?
?
Ķ¼15-20
£Ø1£©ÖĘČ”Ä³ĪŽÉ«·Ē½šŹōŃõ»ÆĪļĘųĢåŹ±£¬×¶ŠĪĘæXÄŚµÄŅ©Ę·Ó¦ŹĒ (””””)?
A.ĶÓėĻ”HNO3”””””””””””” B.ĶÓėÅØHNO3?
C.CaCO3ÓėĻ”H2SO4 D.Na2SO3ÓėÅØŃĪĖį?
£Ø2£©Ļ“ĘųĘæYÖŠĖł×°ŅŗĢåÓ¦ŹĒ£Ø²»ŌŹŠķ·ĒĖ®ŌÓÖŹ»ģŌŚĘųĢåÖŠ£©(””””)?
A.ÅØH2SO4 B.NaOHČÜŅŗ?
C.±„ŗĶNaHSO3ČÜŅŗ D.NaHCO3ČÜŅŗ?
£Ø3£©¼ģŃé¼ÆĘųĘææŚŹĒ·ń¼ÆĀśĘųĢ壬ÖĆÓŚĘææŚµÄĀĖÖ½Ó¦Õ“ÉĻµÄČÜŅŗŹĒ(””””)?
A.BaCl2ČÜŅŗ B.KMnO4ĖįŠŌČÜŅŗ?
C.KIµķ·Ū D.ŹÆ»ŅĖ®?
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com