

 £¬Y
£¬Y £®
£®·ÖĪö £Ø1£©øł¾ŻÓŠ»śĪļµÄ½į¹¹ĢŲµćŹéŠ“½į¹¹¼ņŹ½£¬½ų¶ų×ܽį¹ęĀÉČ·¶Ø·Ö×ÓµÄĶØŹ½£»
£Ø2£©ÖįĻ©ÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬øł¾ŻĻ©ĢžµÄ»ÆѧŠŌÖŹĄ“»Ų“š£»Ō×ӵĹ²ĆęÖŖŹ¶æÉŅŌøł¾Ż¼×Ķ锢ŅŅĻ©”¢±½»·µÄæÕ¼ä½į¹¹Ą“»Ų“š£»
£Ø4£©Ķ¬·ÖŅģ¹¹ĢåŹĒÖø·Ö×ÓŹ½ĻąĶ¬µ«ŹĒ½į¹¹²»ĻąĶ¬µÄÓŠ»śĪļÖ®¼äµÄ»„³Ę£»øł¾ŻÓŠ»śĪļµÄ½į¹¹ĢŲµćČ·¶Ø·Ö×Ó½į¹¹¼ņŹ½£»
£Ø5£©2£¬2-¶ž¼×»łøżĶéŹĒČ²ĢžŗĶĒāĘų¼Ó³ÉµÄ²śĪļ£¬øł¾ŻĶéĢžµÄ½į¹¹¼ņŹ½Č·¶ØČ²ĢžµÄ½į¹¹¼ņŹ½£®
½ā“š ½ā£ŗ£Ø1£©øł¾ŻÓŠ»śĪļµÄ½į¹¹ĢŲµć£ŗ¢Ł¢Ś¢Ū¢ÜµÄ½į¹¹¼ņŹ½·Ö±šŹĒC6H6”¢C8H8”¢C10H10£¬C12H12£¬ĖłŅŌ·Ö×ÓµÄĶØŹ½ŹĒC2nH2n£Øn”Ż3µÄÕūŹż£©£¬ŠżĶéµÄ·Ö×ÓŹ½·Ö±šŹĒ
C9H12”¢C12H16”¢C15H20£¬C18H24£¬ĖłŅŌ·Ö×ÓµÄĶØŹ½ŹĒC3n+6H4n+8£Øn”Ż1£©£»¹Ź“š°øĪŖ£ŗCnHn£ØnŹĒ“óÓŚ»ņŹĒµČÓŚ6µÄżŹż£©£»ŹĒC3n+6H4n+8£Øn”Ż1£©£»
£Ø2£©ÖįĻ©ÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¾ßÓŠĻ©ĢžµÄ»ÆѧŠŌÖŹ£¬»·ĶéĢž²»ÄÜŗĶøßĆĢĖį¼Ų·“Ó¦£¬ADÕżČ·£»Ō×ӵĹ²ĆęÖŖŹ¶æÉŅŌøł¾Ż¼×Ķ锢ŅŅĻ©”¢±½»·µÄæÕ¼ä½į¹¹£¬¹ŹBÕżČ·£¬¹ŹŃ”ABD£»
£Ø4£©Ķ¬·ÖŅģ¹¹ĢåŹĒÖø·Ö×ÓŹ½ĻąĶ¬µ«ŹĒ½į¹¹²»ĻąĶ¬µÄÓŠ»śĪļÖ®¼äµÄ»„³Ę£»ÖįĻ©¢ŁC6H6µÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåX£¬ŗĖ“Ź²ÕńĒāĘ×ÖŠÖ»ÓŠŅ»Öַ壬XŹōÓŚÖ¬»·ĢžĒŅ²»ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«ĖłŅŌŹĒ±½£¬ÖįĻ©¢ŚµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåYŅ²Ö»ÓŠŅ»Öַ壬YŹōÓŚÖ¬»·ĢžĒŅ²»ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬ĖłŅŌŹĒĮ¢·½Ķ飬¹Ź“š°øĪŖ£ŗ £»
£» £»
£»
£Ø5£©2£¬2-¶ž¼×»łøżĶéŹĒČ²ĢžŗĶĒāĘų¼Ó³ÉµÄ²śĪļ£¬øł¾ŻĶéĢžµÄ½į¹¹¼ņŹ½Č·¶ØČ²ĢžµÄ½į¹¹¼ņŹ½Ó¦øĆŹĒ£ŗC£ØCH3£©3CH3CH3CH3C”ŌCH£®¹Ź“š°øĪŖ£ŗC£ØCH3£©3CH3CH3CH3C”ŌCH£®
µćĘĄ ±¾Ģāæ¼²éѧɜ½į¹¹¼ņŹ½µÄŹéŠ“ŅŌ¼°Ķ¬·ÖŅģ¹¹ĢåµÄÅŠ¶Ļ£¬×¢ŅāÖŖŹ¶µÄ¹éÄÉŗĶŹįĄķŹĒ¹Ų¼ü£¬ÄѶČÖŠµČ£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
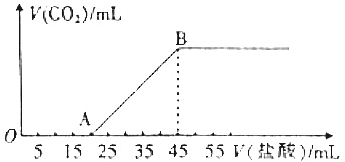
| A£® | 0.2 mol•L-1 µÄH2SO4ČÜŅŗ | |
| B£® | 0.2 mol•L-1 µÄHNO3ČÜŅŗ | |
| C£® | 0.2 mol•L-1 µÄH2SO4ŗĶHNO3»ģŗĻČÜŅŗ | |
| D£® | 1 mol•L-1 µÄH2SO4ŗĶHNO3»ģŗĻČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĘÆ°×·Ū | B£® | ŅŗĀČ | C£® | Ć÷·Æ | D£® | µØ·Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ÄæµÄ | ²Ł×÷ |
| A | ÅäÖĘ100mL 1.0mol/L MgSO4ČÜŅŗ | ½«12.0g MgSO4¹ĢĢåµ¹½ų100mLČŻĮæĘæÖŠ£¬Č»ŗó¼ÓČė100mLÕōĮóĖ® |
| B | Č·¶ØNaClČÜŅŗÖŠŹĒ·ń»ģÓŠNa2CO3 | ȔɣĮæČÜŅŗµĪ¼ÓCaCl2ČÜŅŗ£¬¹Ū²ģŹĒ·ń³öĻÖ°×É«»ė×Ē |
| C | ÖʱøFe£ØOH£©3½ŗĢå | ĻņŹ¢ÓŠ·ŠĖ®µÄÉÕ±ÖŠµĪ¼ÓFeCl3±„ŗĶČÜŅŗ²¢³¤Ź±¼ä¼ÓČČÖó·Š |
| D | Č”³ö·ÖŅŗĀ©¶·ÖŠĖłŠčµÄÉĻ²ćŅŗĢå | ĻĀ²ćŅŗĢå“Ó·ÖŅŗĀ©¶·ĻĀ¶Ė¹ÜæŚ·Å³öŗ󣬹Ų±Õ»īČū£¬»»Ņ»øö½ÓŹÕČŻĘ÷£¬ÉĻ²ćŅŗĢå¼ĢŠų“Ó·ÖŅŗĀ©¶·ĻĀ¶Ė¹ÜæŚ·Å³ö |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com