
 4PCl3(g) + a kJ£¬ P4(s)£«10Cl2(g)
4PCl3(g) + a kJ£¬ P4(s)£«10Cl2(g) 4PCl5(g) + b kJ”£P4¾ßÓŠÕżĖÄĆęĢå½į¹¹£¬PCl5ÖŠP£Cl¼üµÄ¼üÄÜĪŖc kJ/mol£¬PCl3ÖŠP£Cl¼üµÄ¼üÄÜĪŖ1.2c kJ/mol”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
4PCl5(g) + b kJ”£P4¾ßÓŠÕżĖÄĆęĢå½į¹¹£¬PCl5ÖŠP£Cl¼üµÄ¼üÄÜĪŖc kJ/mol£¬PCl3ÖŠP£Cl¼üµÄ¼üÄÜĪŖ1.2c kJ/mol”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ| A£®P£P¼üµÄ¼üÄÜ“óÓŚP£Cl¼üµÄ¼üÄÜ |
B£®æÉĒóCl2(g)£«PCl3(g) PCl5(s)µÄ·“Ó¦ČČ PCl5(s)µÄ·“Ó¦ČČ |
C£®Cl£Cl¼üµÄ¼üÄÜĪŖ 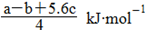 |
D£®P£P¼üµÄ¼üÄÜĪŖ  |
 4PCl3(g) + a kJ£¬¢Ś P4(s)£«10Cl2(g)
4PCl3(g) + a kJ£¬¢Ś P4(s)£«10Cl2(g) 4PCl5(g) + b kJ”£¢Ś£¢ŁÕūĄķæɵĆCl2(g)£«PCl3(g)
4PCl5(g) + b kJ”£¢Ś£¢ŁÕūĄķæɵĆCl2(g)£«PCl3(g) PCl5(g) £«
PCl5(g) £«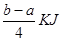 ”£µ«ŹĒƻӊPCl5(g)
”£µ«ŹĒƻӊPCl5(g)  PCl5(s)µÄČČŠ§Ó¦£¬ĖłŅŌ²»ÄÜĒóCl2(g)£«PCl3(g)
PCl5(s)µÄČČŠ§Ó¦£¬ĖłŅŌ²»ÄÜĒóCl2(g)£«PCl3(g) PCl5(s)µÄ·“Ó¦ČČ”£“ķĪó”£C.øł¾Ż·“Ó¦ Cl2(g)£«PCl3(g)
PCl5(s)µÄ·“Ó¦ČČ”£“ķĪó”£C.øł¾Ż·“Ó¦ Cl2(g)£«PCl3(g) PCl5(g) £«
PCl5(g) £«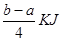 µÄ·“Ó¦ČČÓė¼üÄܵĹŲĻµæɵĆCl£Cl£«3”Į1.2c-5”Įc=
µÄ·“Ó¦ČČÓė¼üÄܵĹŲĻµæɵĆCl£Cl£«3”Į1.2c-5”Įc=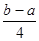 £¬ÕūĄķæɵĆCl£ClµÄ¼üÄÜĪŖ
£¬ÕūĄķæɵĆCl£ClµÄ¼üÄÜĪŖ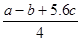 KJ/mol”£ÕżČ·”£D.øł¾Ż¼üÄÜÓė·“Ó¦ČČ¹ŲĻµ °ŃCl£ClµÄ¼üÄÜĪŖ
KJ/mol”£ÕżČ·”£D.øł¾Ż¼üÄÜÓė·“Ó¦ČČ¹ŲĻµ °ŃCl£ClµÄ¼üÄÜĪŖ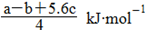 “ųČė¢ŁæɵĆ6P”ŖP+6”Į
“ųČė¢ŁæɵĆ6P”ŖP+6”Į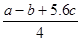 £4”Į3”Į1.2c=a”£½āµĆP£P¼üµÄ¼üÄÜĪŖ
£4”Į3”Į1.2c=a”£½āµĆP£P¼üµÄ¼üÄÜĪŖ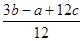 KJ/mol.“ķĪó”£
KJ/mol.“ķĪó”£ 


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®½öÓŠ¢Ś | B£®½öÓŠ¢Ś¢Ü | C£®½öÓŠ¢Ś¢Ū¢Ü | D£®Č«²æ·ūŗĻŅŖĒó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
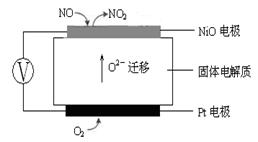
 4NO(g)£«CO2(g)£«2H2O(g) ”÷H1£¼0
4NO(g)£«CO2(g)£«2H2O(g) ”÷H1£¼0 2N2(g)£«CO2(g)£«2H2O(g) ”÷H2£¼0
2N2(g)£«CO2(g)£«2H2O(g) ”÷H2£¼0 N2(g) £«CO2(g) £«2H2O(g) ”÷H3£½ ”££ØÓĆ”÷H1ŗĶ”÷H2±ķŹ¾£©
N2(g) £«CO2(g) £«2H2O(g) ”÷H3£½ ”££ØÓĆ”÷H1ŗĶ”÷H2±ķŹ¾£©| Ķ¶ĮĻ±Č[n(NO2) / n(CH4)] | 400 K | 500 K | 600 K |
| 1 | 60% | 43% | 28% |
| 2 | 45% | 33% | 20% |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 2CO2(g)+2H2O(l)””¦¤H1="-870.3" kJ”¤mol-1
2CO2(g)+2H2O(l)””¦¤H1="-870.3" kJ”¤mol-1 CO2(g)””¦¤H2="-393.5" kJ”¤mol-1
CO2(g)””¦¤H2="-393.5" kJ”¤mol-1 H2O(l)””¦¤H3="-285.8" kJ”¤mol-1
H2O(l)””¦¤H3="-285.8" kJ”¤mol-1 CH3COOH(l)
CH3COOH(l)| A£®¦¤H="+488.3" kJ”¤mol-1 | B£®¦¤H="-244.15" kJ”¤mol-1 |
| C£®¦¤H="-977.6" kJ”¤mol-1 | D£®¦¤H="-488.3" kJ”¤mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 CO2(g) + H2(g)£¬·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹµÄÅضČČēÓŅĶ¼t1Ē°ĖłŹ¾±ä»Æ”£Čō±£³ÖĪĀ¶Č²»±ä£¬t2Ź±ŌŁĻņČŻĘ÷ÖŠ³äČėCO”¢H2ø÷1mol£¬Ę½ŗā½« ŅĘ¶Æ£ØĢī”°Ļņ×ó”±”¢ ”°ĻņÓŅ”±»ņ”°²»”±£©”£t2Ź±£¬Čōøı䷓ӦĢõ¼ž£¬µ¼ÖĀH2ÅØ¶Č·¢ÉśČēÓŅĶ¼t2ŗóĖłŹ¾µÄ±ä»Æ£¬ŌņøıäµÄĢõ¼žæÉÄÜŹĒ £ØĢī·ūŗÅ£©”£
CO2(g) + H2(g)£¬·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹµÄÅضČČēÓŅĶ¼t1Ē°ĖłŹ¾±ä»Æ”£Čō±£³ÖĪĀ¶Č²»±ä£¬t2Ź±ŌŁĻņČŻĘ÷ÖŠ³äČėCO”¢H2ø÷1mol£¬Ę½ŗā½« ŅĘ¶Æ£ØĢī”°Ļņ×ó”±”¢ ”°ĻņÓŅ”±»ņ”°²»”±£©”£t2Ź±£¬Čōøı䷓ӦĢõ¼ž£¬µ¼ÖĀH2ÅØ¶Č·¢ÉśČēÓŅĶ¼t2ŗóĖłŹ¾µÄ±ä»Æ£¬ŌņøıäµÄĢõ¼žæÉÄÜŹĒ £ØĢī·ūŗÅ£©”£


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 CH3CH2OH(g)+3H2O(g) ¦¤H1
CH3CH2OH(g)+3H2O(g) ¦¤H1 CO(g)+3H2(g) ¦¤H2
CO(g)+3H2(g) ¦¤H2| A£®Ōö“óŃ¹Ēæ | B£®¼Ó“߻ƼĮ | C£®Ōö“óCO2µÄÅØ¶Č | D£®¼°Ź±·ÖĄėĢåĻµÖŠµÄŅŅ“¼ |
 CO2(g)+H2(g) ¦¤H3
CO2(g)+H2(g) ¦¤H3 H1”¢
H1”¢ H3±ķŹ¾£©”£
H3±ķŹ¾£©”£
 H2________0£ØĢī”°>”±”¢”°=”±»ņ”°<”±£©”£Ä³ĪĀ¶ČĻĀ£¬ĻņČŻ»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ¼ÓČė1 mol¼×ĶéŗĶ1molĖ®ÕōĘų£¬¾¹ż5h·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±²āµĆCH4µÄÅØ¶Č±äĪŖ0.5 mol/L”£øĆĪĀ¶ČĻĀ£¬·“Ó¦(ii)µÄĘ½ŗā³£ŹżK=__________________£¬·“Ó¦æŖŹ¼ÖĮ“ļµ½Ę½ŗāŹ±ĒāĘųµÄ·“Ó¦ĖŁĀŹv(H2)=_________”£
H2________0£ØĢī”°>”±”¢”°=”±»ņ”°<”±£©”£Ä³ĪĀ¶ČĻĀ£¬ĻņČŻ»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ¼ÓČė1 mol¼×ĶéŗĶ1molĖ®ÕōĘų£¬¾¹ż5h·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±²āµĆCH4µÄÅØ¶Č±äĪŖ0.5 mol/L”£øĆĪĀ¶ČĻĀ£¬·“Ó¦(ii)µÄĘ½ŗā³£ŹżK=__________________£¬·“Ó¦æŖŹ¼ÖĮ“ļµ½Ę½ŗāŹ±ĒāĘųµÄ·“Ó¦ĖŁĀŹv(H2)=_________”£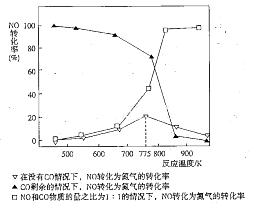
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 H2(g)£«CO2(g)””¦¤H2£»
H2(g)£«CO2(g)””¦¤H2£» CO(g)£«H2(g)””¦¤H3£»
CO(g)£«H2(g)””¦¤H3£» 2CO(g)£«4H2O(g)
2CO(g)£«4H2O(g)²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 CO(g)£«H2(g)””¦¤H£½£«131.3 kJ”¤mol£1£¬
CO(g)£«H2(g)””¦¤H£½£«131.3 kJ”¤mol£1£¬| A£®ÉżøßĪĀ¶Č | B£®Ōö¼ÓĢ¼µÄÓĆĮæ | C£®¼ÓČė“߻ƼĮ | D£®ÓĆCOĪüŹÕ¼Į³żČ„CO |
 2CO(g)””¦¤H£½£«172.5 kJ”¤mol£1£¬ŌņCO(g)£«H2O(g)
2CO(g)””¦¤H£½£«172.5 kJ”¤mol£1£¬ŌņCO(g)£«H2O(g) CO2(g)£«H2(g)µÄģŹ±ä¦¤H£½________”£
CO2(g)£«H2(g)µÄģŹ±ä¦¤H£½________”£ CH3OH(g)”£¼×“¼ŹĒŅ»ÖÖČ¼ĮĻ£¬æÉĄūÓĆ¼×“¼Éč¼ĘŅ»øöČ¼ĮĻµē³Ų£¬ÓĆĻ”ĮņĖį×÷µē½āÖŹČÜŅŗ£¬¶ąæ׏ÆÄ«×÷µē¼«£¬øƵē³Ųøŗ¼«·“Ó¦Ź½ĪŖ__________________________________”£
CH3OH(g)”£¼×“¼ŹĒŅ»ÖÖČ¼ĮĻ£¬æÉĄūÓĆ¼×“¼Éč¼ĘŅ»øöČ¼ĮĻµē³Ų£¬ÓĆĻ”ĮņĖį×÷µē½āÖŹČÜŅŗ£¬¶ąæ׏ÆÄ«×÷µē¼«£¬øƵē³Ųøŗ¼«·“Ó¦Ź½ĪŖ__________________________________”£ CO2(g)£«H2(g)”£µĆµ½ČēĻĀŹż¾Ż£ŗ
CO2(g)£«H2(g)”£µĆµ½ČēĻĀŹż¾Ż£ŗ| ĪĀ¶Č/”ę | ĘšŹ¼Įæ/mol | Ę½ŗāĮæ/mol | “ļµ½Ę½ŗāĖłŠčŹ±¼ä/min | ||
| H2O | CO | H2 | CO | | |
| 900 | 1.0 | 2.0 | 0.4 | 1.6 | 3.0 |
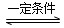 2NH3(g)””¦¤H£½£92.4 kJ”¤mol£1”£ŹµŃéŹŅÄ£Äā»Æ¹¤Éś²ś£¬·Ö±šŌŚ²»Ķ¬ŹµŃéĢõ¼žĻĀ·“Ó¦£¬N2ÅضČĖꏱ¼ä±ä»ÆČēĶ¼¼×ĖłŹ¾”£
2NH3(g)””¦¤H£½£92.4 kJ”¤mol£1”£ŹµŃéŹŅÄ£Äā»Æ¹¤Éś²ś£¬·Ö±šŌŚ²»Ķ¬ŹµŃéĢõ¼žĻĀ·“Ó¦£¬N2ÅضČĖꏱ¼ä±ä»ÆČēĶ¼¼×ĖłŹ¾”£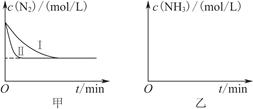
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com