
LiNi0.25Co0.75O2������ӵ�ص�һ�ָ����ܵĶ�Ԫ�������Բ��ϣ����Ʊ�ԭ���ɱ�ʾΪ4Ni0.25Co0.75(OH)2��4LiOH��O2===4LiNi0.25Co0.75O2��6H2O(��֪Ni��Co�Ļ��ϼ۾��У�2�ͣ�3)������˵������ȷ����(����)
A��Ni0.25Co0.75(OH)2��Ni�Ļ��ϼ��ǣ�2
B��LiNi0.25Co0.75O2��Co�Ļ��ϼ��ǣ�3
C���÷�Ӧ��LiOH�ǻ�ԭ��
D���÷�Ӧ��O2��������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ȥ�����е�Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�����
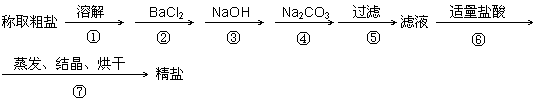
��1���ж�BaCl2�ѹ����ķ����� ��
��2���ڢܲ��У�д����Ӧ�Ļ�ѧ����ʽ���������Һ��Ca2+����Ҫ������ʽΪCaCl2��
��3�������������ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
(4)�ڡ������ᴿ����ʵ���У�����õ������������ܽ⡢���ˡ��������������ж��õ�����������д�������ʹ�õ�������������__________ __
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
100 g̿��ȼ�����������У�COռ2/3��CO2ռ1/3����C(s)��1/2O2(g)===CO(g)����H����110.35 kJ/mol��CO(g)��1/2O2(g)===CO2(g)����H����282.57 kJ/mol������Щ̿����ȫȼ�������ʧ��������(����)
A��784.92 kJ B��2 489.44 kJ
C��1 569.83 kJ D��3 274.3 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(BN)�����ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԡ����ǵľ���ṹ����ͼ��ʾ��
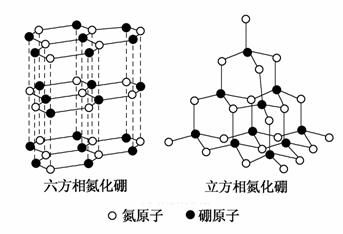
(1)��̬��ԭ�ӵĵ����Ų�ʽΪ________��
(2)���������־����˵������ȷ����________(��ѡ����ĸ)��
a�������൪�����ЦҼ��ͦм�������Ӳ�ȴ�
b�������൪������������С�������ʵ���
c�����־����е�B��N����Ϊ���ۼ�
d�����־����Ϊ���Ӿ���
(3)�����൪���������һ����ԭ�������ڵ�ԭ�ӹ��ɵĿռ乹��Ϊ________����ṹ��ʯī����ȴ�����磬ԭ����_________________��
(4)�����൪�������У���ԭ�ӵ��ӻ��������Ϊ__________���þ������Ȼ��������ظ�ԭ����Լ300 km�Ĺŵؿ��б����֡�������һ�����γ���ʵ���ƶ�ʵ�����������൪����ϳ������൪������Ҫ������Ӧ��______________________________________________________________��
(5)NH4BF4(�������)�Ǻϳɵ��������ܵ�ԭ��֮һ��1 mol NH4BF4����________mol��λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(�Ҷ���)������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ�����(��2���ᾧˮ)�Ĺ����������£�

�ش��������⣺
(1)CO��NaOH��һ�������ºϳɼ����ơ������Ƽ�������Ļ�ѧ��Ӧ����ʽ�ֱ�Ϊ________________��________________��
(2)���Ʊ������������ι��˲��������˲����ٵ���Һ��________��������________�����˲����ڵ���Һ��________��________��������________��
(3)���չ����Тۺܵ͢�Ŀ����_________________________________��
(4)���˽�������������ֱ���������ữ�Ʊ����ᡣ�÷�����ȱ���Dz�Ʒ���������к��е�������Ҫ��__________________��
(5)�ᾧˮ�ϲ����Ʒ�Ĵ����ø�����ط��ⶨ�����������Ʒ0.250 g����ˮ����0.050 0 mol��L��1������KMnO4��Һ�ζ�����dz�ۺ�ɫ�����ʣ�����KMnO4��Һ15.00 mL����Ӧ�����ӷ���ʽΪ______________________����ʽ����ó�Ʒ�Ĵ���____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ��������ص����ӷ���ʽ����ȷ����(����)
A��̼��Ʒ�ĩ�м��������Һ���������٣�������ɫ���壺CaCO3��2H��===Ca2����CO2����H2O
B����BaCl2��Һ��ͨ��SO2���壬���ְ�ɫ������Ba2����SO2��H2O===BaSO3����2H��
C����H2O2��Һ�еμ��ữ��KMnO4��Һ��KMnO4��Һ��ɫ��2MnO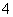 ��7H2O2��6H��===2Mn2����6O2����10H2O
��7H2O2��6H��===2Mn2����6O2����10H2O
D���������ʵ���Ũ�ȡ��������Ba(OH)2��Һ��NaHSO4��Һ��ϣ����ɰ�ɫ������Ba2����SO ��H����OH��===BaSO4����H2O
��H����OH��===BaSO4����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
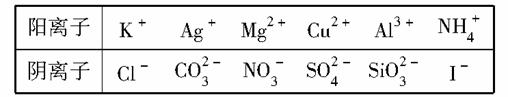
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������________��
(2)���м�������������ɫ��������ӷ���ʽ��________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������______�����ݴ��Ʋ�ԭ��ҺӦ�ó�________�ԣ�ԭ����________________________
(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ________________����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1 mol CnHmO2���л�����O2����ȫȼ�պ����ɵ�CO2��H2O(g)�������ȣ�������44.8 L O2(��״��)�����л��������Cԭ����nΪ(����)
A��1 B��2
C��3 D��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˽�����K2SO4��MgSO4��KNO3�����ᴿ�����Ƶô�����KNO3��Һ ��ijѧ���������ʵ�鷽����
��ijѧ���������ʵ�鷽����

(1)������Ϊ____________________��
(2)�����ڡ��ܼ�����Լ����ο���Ϊ____________________________________��
(3)����ж�SO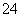 �ѳ�����_____________________________________________��
�ѳ�����_____________________________________________��
(4)ʵ������в����Ķ�γ���________(���Ҫ������Ҫ��)��ι��ˣ���������
_____________ ____________________________________________
____________________________________________ _______________��
_______________��
(5)��ͬѧ��ʵ����Ʒ����Ƿ����ܣ���˵�����ɣ�___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com