
��1�������ʵ�����NO2��N2O���ڱ�״���µ����֮�� ������Oԭ�Ӹ���֮�� �����ߵ�����֮�� ��
��2��ͬ�¡�ͬѹ�£���������CH4��H2��O2��N2�������壬���������_ _ __�������� ������___ _���ܶ�������__ __��ԭ����������__ __�����ѧʽ��
������___ _���ܶ�������__ __��ԭ����������__ __�����ѧʽ��
��3��NaCl��MgCl2��AlCl3������Һ�������ʵ���Ũ����ͬ����������Һ�������ӵ����ʵ���Ũ��֮��Ϊ ���������ͬ�����ʵ���Ũ��֮��Ϊ1��2��3����������Һ�������ӵ����ʵ���Ũ��֮�� ���������ͬ�����ʵ���Ũ����ͬ��AgNO3��Һ�����Ũ������������Һǡ�÷�Ӧ��������������Һ�����֮��Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
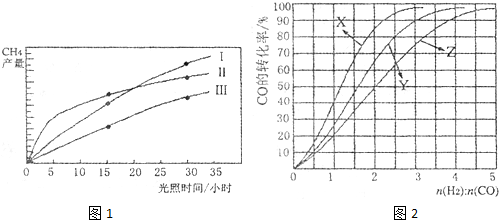
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��
(1)��O〜30Сʱ�ڣ�CH4��ƽ������������
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g)CO(g)+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3)�÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ�����ֱ�Ϊ
��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g) CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
��2700C��ʵ��������Ӧ��������_________(����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________(����ĸ����
A. 1 〜1.5 B. 2. 5〜3 C. 3. 5〜4. 5
(5)ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ������
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��
(1) ��O?30Сʱ�ڣ�CH4��ƽ���������� ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g) CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3) �÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ���� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g)  CH3OH (g), ��H<0�ϳɼ״�����230��C?270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
CH3OH (g), ��H<0�ϳɼ״�����230��C?270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
��2700C��ʵ��������Ӧ��������_________ (����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________ (����ĸ����
A. 1 ?1.5 B. 2. 5?3 C. 3. 5?4. 5
(5) ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ������
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��

(1)
��O〜30Сʱ�ڣ�CH4��ƽ���������� ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g) CO(g)
+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g)
+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3)
�÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ���� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g)
 CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
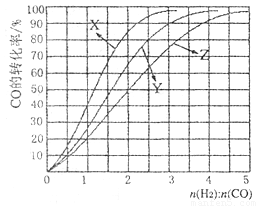
��2700C��ʵ��������Ӧ��������_________ (����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________ (����ĸ����
A. 1 〜1.5 B. 2. 5〜3 C. 3. 5〜4. 5
(5) ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com