
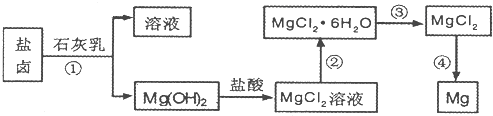
��9�֣�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ�ѧ�����������չ�������۵ġ���֪��ˮ��þ����Ҫ�������£�

ѧ�����������������������⣺
(һ)�ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵĸ�����
������ѧ������Լ��Ĺ۵㡣
ѧ���Ĺ۵㣺ֱ������ˮ�м����������
ѧ���ҵĹ۵㣺���¼���������ˮ���ټ����������
ѧ�����Ĺ۵㣺����ɹ�κ�Ŀ�±ˮ���ټ������������
������������ѧ������Ĺ۵��Ƿ���ȷ�����ǻ�����������ɡ�
|
| �Ƿ���ȷ | �������� |
| ѧ���Ĺ۵� |
|
|
| ѧ���ҵĹ۵� |
|
|
| ѧ�����Ĺ۵� |
|
|
(��)�ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵķ��룿
��1��Ϊ��ʹþ���ӳ�������������������Լ����������������������ѧʽ����
��2������������Լ����������������������ѧʽ����
��3���Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ������������˵�ұþ������ ��
A. 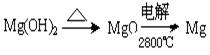
B. ![]()
C. 
D. 

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
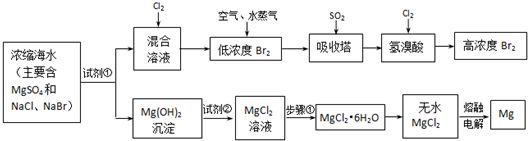
| ||
| ||
 MgO+2 HCl��+5H2O��
MgO+2 HCl��+5H2O�� MgO+2 HCl��+5H2O��
MgO+2 HCl��+5H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� | �Ƿ���ȷ | �������� |
| ����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
| ����2�����¼���������ˮ���ټ�������� | ������ | ������ |
| ����Ϊ����������������ǣ����ģ� | ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �Ƿ���ȷ | �������� | |
| ѧ��1�Ĺ۵� | �� �� |
��ˮ��þ����Ũ��С��������������������þ���ӵij��� ��ˮ��þ����Ũ��С��������������������þ���ӵij��� |
| ѧ��2�Ĺ۵� | �� �� |
��Դ���Ĵ�ˮ���ۺ����õͣ��ɱ��� ��Դ���Ĵ�ˮ���ۺ����õͣ��ɱ��� |
| ѧ��3�Ĺ۵� | �� �� |
þ���Ӹ���Ũ�ȸߣ��ɱ��� þ���Ӹ���Ũ�ȸߣ��ɱ��� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
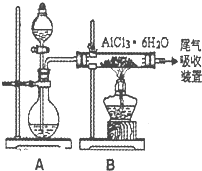
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com