
ijͬѧ��ѧϰ����������ʱ������������ͭ�ķ�Ӧ��������о�����������и��⡣
��1���ڼס��������ձ��У��ֱ�װ��40mLŨ�Ⱦ�Ϊ2mol��L��1��ϡ�����ϡ���ᣬ�������и����� 4g��״ͭ˿���۲��������������ʵ�鱨�棺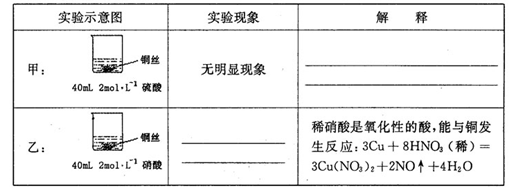
��2����ַ�Ӧ���ס����ձ���ϣ���ʹ֮��ַ�Ӧ������������Һ����Ϊ____ ��ʣ�����������Ϊ g
��3��������������Һ���V��V>40mL���ɱ䣬�������ݲ��䣬��
�ٵ��ס����ձ���ϳ�ַ�Ӧ����Һ��ֻ��һ������ʱ��V=____ mL����Ҫ����Һ�е�Cu2+������ȫ��Ӧ��NaOHʹ��Һ��pH����Ϊ____ ����֪KsP[Cu��OH��2]=2.2��l0��20,1g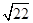 =0.7��
=0.7��
���ܷ�ͨ��������Һ����ĸı䣬ʹͭ˿�ڼס����ձ���ϳ�ַ�Ӧ����ȫ�ܽ�? ��д����������________ ��
26����1���ף�ϡ����ֻ�ܱ�����������ԣ���ͭ���ڽ����˳�����֮���ܽ����û�����
��1�֣��� �ң�ͭ˿���ܽ⣬ͭ˿���������ݲ�������Һ��ɫ������1�֣���
��2��CuSO4��Cu(NO3)2��2�֣���2.24��2�֣���3����60��2�֣� 6.7��2�֣��ڷ�1�֣�����3�֣�
���������������1���ף�����ϡ����ֻ�ܱ�����������ԣ���ͭ���ڽ����˳�����֮�����Բ��ܽ����û��������ң������������������ᣬ����������ͭ�����ʵ��������ͭ˿���ܽ⣬ͭ˿���������ݲ�������Һ��ɫ������
��2���������������ʵ�����0.04L��2mol/L��0.08mol������NO3�������ʵ�����0.08mol�������ӵ����ʵ�����0.24mol�����ݷ���ʽ3Cu+8H++2NO3��=3Cu2++2NO��+4H2O��֪�����Ӳ��㣬NO3���������������ܽ�ͭ��������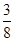 ��0.24mol��64g/mol��5.76g��8g������ͭ������ʣ��ͭ��������8g��5.76g��2.24g��1������Һ������������Һ����ΪCuSO4��Cu(NO3)2��
��0.24mol��64g/mol��5.76g��8g������ͭ������ʣ��ͭ��������8g��5.76g��2.24g��1������Һ������������Һ����ΪCuSO4��Cu(NO3)2��
��3��������Һ������ֻ��һ�֣��������Ӧ��������ͭ���������ӷ���ʽ3Cu��8H+��2NO3��=3Cu2+��2NO����4H2O��֪����Ҫ�����ӵ����ʵ�����0.08mol��4��0.032mol�����������ṩ����������0.032mol��0.08mol��0.24mol��������������ʵ�����0.24mol��2��0.12mol����������Һ�������0.12mol��2mol/L��0.06L��60ml������Һ��ͭ����Ũ��С�ڻ����10��5mol/Lʱ���Կ���ͭ������ȫ������������ܶȻ�������֪����ʱ��Һ��c(OH��)��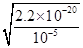 ��
��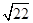 ��10��8mol/L����c(H+)��
��10��8mol/L����c(H+)��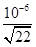 �����pH��6��1g
�����pH��6��1g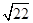 ��6.7��
��6.7��
�����ձ����е�NO3����40mL��10-3L/mL��2mol/L��0.08mol�������ӷ���ʽ3Cu��8H+��2NO3��=3Cu2+��2NO����4H2O������������Cu������Ϊ0.08mol��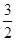 ��64g/mol��7.68g��С�����ձ���ͭ˿����������������ͭ�ܽ��ꡣ
��64g/mol��7.68g��С�����ձ���ͭ˿����������������ͭ�ܽ��ꡣ
���㣺����ͭ�����ᡢ��������ʣ�ͭ�����ᷴӦ�ļ��㣻�ܶȻ�������Ӧ�õ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʵ�������ö������̸�Ũ���ᷴӦ�Ʊ����﴿��������������������ͼ��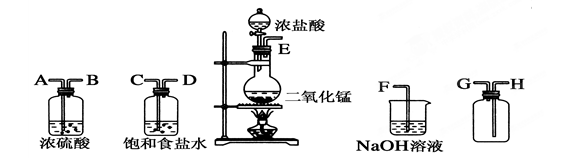
(1)����������������ȷ˳��(����ӿڴ�����ĸ)��__��__��__��__��__��__��__��__����2�֣�
(2)װ���У�����ʳ��ˮ��������______________��NaOH��Һ��������____________����4�֣�
(3)д�����л�ѧ��Ӧ�ķ���ʽ��
�����巢��װ���н��еķ�Ӧ��________________________________________����2�֣�
��NaOH��Һ�з����ķ�Ӧ��__________________________________________ ����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ����ȡ��ˮ�Ȼ�ͭ��ʵ��װ��ͼ����Ũ����μӵ�ʢ�ж������̷�ĩ��Բ����ƿ�С���ش��������⣺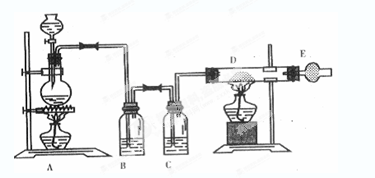
(1)ʢ��Ũ�������������Ϊ�ߣߣߣ���
(2)��ƿ�з�����Ӧ�Ļ�ѧ����ʽ ��
(3)Cƿ�е��Լ����ߣߣߣ������������ߣߣߣ���
(4)������D�з�����Ӧ�Ļ�ѧ����ʽ ����Ӧ�������ߣߣ���
(5)�����E��ʢ�м�ʯ��(CaO+NaOH)�����������ߣߣߣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棬���������۵�Ϊ231�档װ��A�з�Ũ����B�з�MnO2�����������������������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ������˷�Ӧ���̷ų��������ȣ�����ش����и����⡣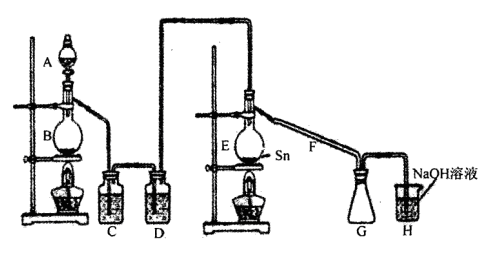
��1������ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��H�з�Ӧ�����ӷ���ʽ��_________________________________________________��
E�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��3��C��D�е��Լ��ֱ���_______________��____________________��
��4������A��B�����Ʒֱ���_____________��____________��F��������_____________��
��5��ʵ��ʱӦ�ȵ�ȼ_________���ƾ��ƣ������¶�Ӧ����________ �棬��________������ֹͣ���ȡ�
��6����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________________________________________________��
��7���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ����С��ͬѧ���ʵ�飬̽��ľ̿��Ũ�����ڼ��������·�Ӧ��������ijɷ֡�
��ʵ��̽����
��1��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��C+2H2SO4��Ũ�� CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
��2��Aװ����Ʒ����Һ��ɫ �����ɫ������ɫ������֤������ ���塣
��3��ʵ������У�װ��C���۲쵽��������_______________________________��
��ʵ�����ۡ�
��4����ͬѧ��Bװ���ܷ����SO2���������塣����ΪӦ����B��Cװ��֮��������ͼ�� װ�ã���ȷ��SO2�Ƿ������
����ϵʵ�ʡ�
��5��ú��ʯ�͵�ȼ�չ����ж��ж�������Ͷ�����̼�ŷţ����ж���������ɵĻ���Ӱ����Ҫ��_________��������̼��ɵĻ���Ӱ����Ҫ��_______����ÿ�ո�ֻ��һ��ѡ�
A������ B���ƻ������� C������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪Na2SO3����������ɷ�����Ӧ��Na2SO3��H2SO4=Na2SO4��H2O��SO2������ͼ��ʵ������ȡSO2����֤SO2��ijЩ���ʵ�װ��ͼ���Իش� [
[
��1�����е�ʵ������Ϊ��ɫʯ����Һ_________________����ʵ��֤��SO2��________���塣
��2�����е�Ʒ����Һ___________________��֤��SO2��__________�ԡ�
��3�����е�ʵ��������_________________��֤��SO2��__________�ԡ�
��4�����е�ʵ��������_________________��֤��SO2��__________�ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧ��ȤС���ij������ʯ����Ҫ �ɷ�ΪFeS2)������Ԫ�غ����ⶨ��ʵ��̽������ҵ���������̽����
I ����m1,g�û�������Ʒ�������в������������������ͼ��ʾװ�ã��гֺͼ���װ�� ʡ�ԣ���ʯӢ����,��a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2 + 11O2 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2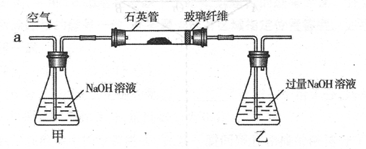
II��Ӧ������,����ƿ�е���Һ�������´���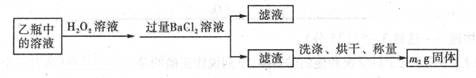
��1��I�У���ƿ�ڷ�����Ӧ�����ӷ���ʽ��____________��__________��
��2��II�У�����H2O2��Һ��������������____________________________
��3���û�����ʯ����Ԫ�ص���������Ϊ____________________________
��4�������ڴ���Ӧ���������Ƚ�������Ŀ�ģ�______________��
��5����ҵ�����г��ð��������ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ��,�� ������ѧ����ʽ��ʾ�䷴Ӧԭ��_______________��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ��Ӧ����ͼ��ʾ��װ�����о����ʵ����ʣ���������X����Ҫ�ɷ��������������ǿ�����ˮ����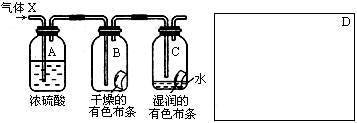
�ش��������⣺
(1)�����о�(ʵ��)����ҪĿ���� ��
(2)ŨH2SO4�������� �����о�Ŀ��ֱ����ص�ʵ�������� ��
(3)��ʵ��װ������ϴ��ڵ�ȱ��Ϊ ��������ͼ��D�������ܿ˷���ȱ�ݵ�װ�á�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com