
ÓĆѧ¹żµÄÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā
(1) ÓĆŅ»ÖÖŹŌ¼Į½«ĻĀĮŠø÷×éĪļÖŹ¼ų±šæŖ£®
¢Ł ŗĶ
ŗĶ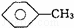 £ŗ”” ””
£ŗ”” ””
¢Ś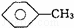 £¬CCl4ŗĶŅŅ“¼£ŗ”” ””
£¬CCl4ŗĶŅŅ“¼£ŗ”” ””
(2) ¶ž¼×±½±½»·ÉĻµÄŅ»äå“śĪļÓŠ6ÖÖĶ¬·ÖŅģ¹¹Ģ壬ÕāŠ©Ņ»äå“śĪļÓėÉś³ÉĖüµÄ¶ŌÓ¦¶ž¼×±½µÄČŪµć·Ö±šĮŠ±ķČēĻĀ£ŗ
| Ņ»äå“ś¶ž¼×±½ | 234”ćC | 206”ćC | 213.8”ćC | 204”ćC | 214.5”ćC | 205”ćC |
| ¶ŌÓ¦¶ž¼×±½ | 13”ćC | £54”ćC | £27”ćC | £54”ćC | £27”ćC | £54”ćC |
ÓɱķÄŚŹż¾ŻæÉŅŌĶʶĻ£ŗ
¢ŁČŪµćĪŖ234”ćCµÄŅ»äå“ś¶ž¼×±½µÄ½į¹¹¼ņŹ½ĪŖ £¬
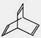 ¢ŚČŪµćĪŖ£54”ćCµÄ¶ž¼×±½µÄĆū³ĘĪŖ ”£
¢ŚČŪµćĪŖ£54”ćCµÄ¶ž¼×±½µÄĆū³ĘĪŖ ”£
(3) ÓŠ»śĪļXµÄ¼üĻߏ½ĪŖ£ŗ £¬·¼ĻćĢžYŹĒXµÄĶ¬·ÖŅģ¹¹Ģ壬YÄÜŹ¹äåĖ®ĶŹÉ«”£
¢ŁYµÄ½į¹¹¼ņŹ½ĪŖ£ŗ ”£
¢ŚYæÉŅŌÓĆĄ“ŗĻ³ÉŅ»ÖÖ¾ŪŗĻĪļ---ÅŻÄĖÜĮĻ£¬ĒėŠ“³öøĆ¾ŪŗĻĪļµÄ½į¹¹¼ņŹ½_ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀ±ķĮŠ³öĮĖijĄäŌž³§ÅŷŵķĻĖ®ÖŠø÷³É·ÖµÄŗ¬Įæ¼°¹ś¼Ņ»·±£±ź×¼ÖµµÄÓŠ¹ŲŹż¾Ż£ŗ
| ĄäŌžŗ¬Šæ ·ĻĖ®Ė®ÖŹ | ¾“¦ĄķŗóµÄĖ®¹ś ¼Ņ»·±£±ź×¼Öµ | |
| Zn2£«ÅضČ/(mg·L£1) | ”Ü800 | ”Ü3.9 |
| pH | 1”«5 | 6”«9 |
| SO | ӆ23 000 | ӆ150 |
¾Ä³Ņ»¹¤ŅÕ“¦ĄķŗóµÄ·ĻĖ®pH£½8£¬³£ĪĀĻĀ£¬øĆ·ĻĖ®ÖŠZn2£«µÄÅضČĪŖ____________mg·
L£1(³£ĪĀĻĀ£¬Ksp[Zn(OH)2]£½1.2”Į10£17)£¬________(Ģī”°·ūŗĻ”±»ņ”°²»·ūŗĻ”±)¹ś¼Ņ»·±£±ź×¼”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°“ŅŖĒóĢīæÕ£ŗ
£Ø1£©£Ø9·Ö£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢ŁŹµŃéŹŅÖĘČ”°±Ęų ___________________________________________
¢ŚÅØĻõĖįµÄ¼ū¹ā·Ö½ā_______________________________________
¢Ū¶žŃõ»ÆµŖŗĶĖ®·“Ó¦_______________________
£Ø2£©(10·Ö)A”¢B”¢C”¢DĪŖ֊ѧ³£¼ūĪļÖŹĒŅ¾łŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬Ļą»„×Ŗ»Æ¹ŲĻµČēĶ¼(·“Ó¦Ģõ¼ž¼°ĘäĖūĪļÖŹŅŃ¾ĀŌČ„)£ŗ
A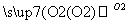 B
B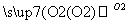 C
C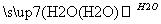 D
D
¢ŁČōA”¢B”¢C”¢D¾łĪŖ»ÆŗĻĪļ£¬¶ųĒŅĖüĆĒµÄĖ®ČÜŅŗ¾łÄÜŹ¹ŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½±äŗģ£¬ŌņDĪŖ(ĢīŠ“»ÆѧŹ½)£ŗ______”£Š“³öB”śCµÄ»Æѧ·½³ĢŹ½£ŗ_____________”£
¢ŚČōAµÄĖ®ČÜŅŗÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬DµÄĻ”ČÜŅŗÄÜŹ¹ŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½±äŗģ”£ŌņDµÄ»ÆѧŹ½ĪŖ______”£Š“³öA”śBµÄ»Æѧ·½³ĢŹ½£ŗ ________________”£
¢ŪČōAĪŖµ„ÖŹ£¬×é³ÉŌŖĖŲµÄŌ×ÓĖłŗ¬ÖŹ×ӵďżÄæŠ”ÓŚ18£¬DĪŖĒæ¼ī£¬Š“³öC”śDµÄ»Æѧ·½³ĢŹ½£ŗ___________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ»ś·“Ó¦ÖŠ£¬·“Ó¦ĄąŠĶĻąĶ¬µÄŹĒ£Ø £©
¢ŁCH3CH£½CH2£«Br2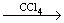 CH3CHBrCH2Br
CH3CHBrCH2Br
¢ŚCH3CH2OH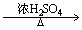 CH2£½CH2£«H2O
CH2£½CH2£«H2O
¢ŪCH3COOH£«CH3CH2OH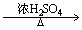 CH3COOCH2CH3£«H2O
CH3COOCH2CH3£«H2O
¢ÜC6H6£«HNO3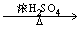 C6H5NO2£«H2O
C6H5NO2£«H2O
A.¢Ł¢Ś B.¢Ū¢Ü C.¢Ł¢Ū D.¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²é“¦¾Ęŗó¼ŻŹ»²ÉÓƵĔ°±ćŠÆŹ½ŅŅ“¼²āĮæŅĒ”±ŅŌČ¼ĮĻµē³ŲĪŖ¹¤×÷ŌĄķ£¬ŌŚĖįŠŌ»·¾³ÖŠ,ĄķĀŪÉĻŅŅ“¼æÉŅŌ±»ĶźČ«Ńõ»ÆĪŖCO2£¬µ«Źµ¼ŹŅŅ“¼±»Ńõ»ÆĪŖX£¬ĘäÖŠŅ»øöµē¼«µÄ·“Ó¦Ź½ĪŖ£ŗCH3CH2OH£2e-”śX+2H+”£ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ£Ø £©
A£®µē³Ų×Ü·“Ó¦ĪŖ£ŗ2CH3CH2OH+O2”ś2CH3CHO+2H2O
B£®ĮķŅ»¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗO2 + 4e- + 2H2O = 4OH-
C£®ŅŅ“¼ŌŚÕż¼«·¢Éś·“Ó¦£¬µē×Ó¾¹żĶāµēĀ·Į÷Ļņøŗ¼«
D£®µē³ŲÄŚ²æH+ÓÉÕż¼«Ļņøŗ¼«ŅʶÆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĖłÓŠ³¬ŹŠ”¢ÉĢ³””¢¼ÆĆ³ŹŠ³”µČÉĢĘ·ĮćŹŪ³”ĖłŹµŠŠĖÜĮĻ¹ŗĪļ“üÓŠ³„Ź¹ÓĆÖĘ¶Č£¬Ņ»Āɲ»µĆĆā·ŃĢį¹©ĖÜĮĻ¹ŗĪļ“ü”£ŌŚČ«¹ś·¶Ī§ÄŚ½ūÖ¹Éś²ś”¢ĻśŹŪ”¢Ź¹ÓĆŗń¶ČŠ”ÓŚ0.025ŗĮĆ×µÄĖÜĮĻ¹ŗĪļ“ü(¼ņ³Ę³¬±”ĖÜĮĻ¹ŗĪļ“ü)”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®ŌŚĖłÓŠ³¬ŹŠ”¢ÉĢ³””¢¼ÆĆ³ŹŠ³”µČÉĢĘ·ĮćŹŪ³”ĖłŹµŠŠĖÜĮĻ¹ŗĪļ“üÓŠ³„Ź¹ÓĆÖĘ¶Č£¬Ö÷ŅŖÄæµÄŹĒæŲÖĘĖÜĮĻÖĘĘ·µÄŹ¹ÓĆ£¬¼õÉŁ”°°×É«ĪŪČ¾”±
B£® ĢåŹĒ±½ŅŅĻ©
ĢåŹĒ±½ŅŅĻ©
C£®¾ŪĀČŅŅĻ©ĖÜĮĻĒæ¶Č“ó£¬æ¹øÆŹ“ŠŌĒ棬æÉŅŌÓĆĄ“°ü×°Šč³¤Ź±¼ä±£“ęµÄŹ³Ę·
D£®ÓĆÓŚŹ³Ę·°ü×°µÄĖÜĮĻÖĘĘ·£¬ŹōÓŚČČĖÜŠŌĖÜĮĻ£¬æÉ»ŲŹÕŌŁĄūÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹĒijµ°°×ÖŹµÄ½į¹¹Ę¬¶Ī£ŗ
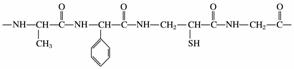
(1)ÉĻŹöµ°°×ÖŹ½į¹¹Ę¬¶ĪµÄĖ®½ā²śĪļÖŠ²»ŹōÓŚ¦Į°±»łĖįµÄ½į¹¹¼ņŹ½ĪŖ________________________________________________________________________”£
(2)ÉĻŹöµ°°×ÖŹ½į¹¹Ę¬¶ĪĖ®½āŗóµÄ°±»łĖįÖŠ£¬Ä³°±»łĖįĢ¼ĒāŌ×ÓŹż±ČÖµ×ī“ó”£
¢ŁøĆ°±»łĖįÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________
________________________________________________________________________ӣ
¢ŚøĆ°±»łĖįĮ½·Ö×ÓĖõŗĻŠĪ³É»·×“½į¹¹ĪļÖŹµÄ·Ö×ÓŹ½ĪŖ
________________________________________________________________________ӣ
¢ŪøĆ°±»łĖįµÄĶ¬·ÖŅģ¹¹ĢåÖŠ£¬ŹōÓŚĻõ»ł»ÆŗĻĪļĒŅ±½»·ÉĻÖ»ÓŠ¼×»łµÄĶ¬·ÖŅģ¹¹ĢåÓŠ________ÖÖ”£
(3)ŅŃÖŖÉĻŹöµ°°×ÖŹ½į¹¹Ę¬¶ĪµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ364£¬ŌņĖ®½āÉś³ÉµÄø÷ÖÖ°±»łĖįµÄĻą¶Ō·Ö×ÓÖŹĮæÖ®ŗĶĪŖ__________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÉĻ³£ÓĆČ¼ÉÕ·ØČ·¶ØÓŠ»śĪļµÄ×é³É”£ÕāÖÖ·½·ØŹĒŌŚµēĀƼÓČČŹ±ÓĆ“æŃõĘųŃõ»Æ¹ÜČѳʷ£¬øł¾Ż²śĪļµÄÖŹĮæČ·¶ØÓŠ»śĪļµÄ×é³É”£ĻĀĶ¼ĖłŹ¾×°ÖĆŹĒÓĆČ¼ÉÕ·ØČ·¶ØÓŠ»śĪļ·Ö×ÓŹ½³£ÓƵÄ×°ÖĆ”£
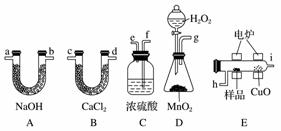
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)²śÉśµÄŃõĘų°““Ó×óµ½ÓŅĮ÷Ļņ£¬ĖłŃ”×°ÖĆø÷µ¼¹ÜµÄĮ¬½ÓĖ³ŠņŹĒ___________”£
(2)C×°ÖĆÖŠÅØH2SO4µÄ×÷ÓĆŹĒ_______________________________________”£
(3)D×°ÖĆÖŠMnO2µÄ×÷ÓĆŹĒ__________________________________________”£
(4)Č¼ÉÕ¹ÜÖŠCuOµÄ×÷ÓĆŹĒ___________________________________________”£
(5)Čō×¼Č·³ĘČ”0.90 gѳʷ(Ö»ŗ¬C”¢H”¢OČżÖÖŌŖĖŲÖŠµÄĮ½ÖÖ»ņČżÖÖ)£¬¾³ä·ÖČ¼ÉÕŗó£¬A¹ÜÖŹĮæŌö¼Ó1.32 g£¬B¹ÜÖŹĮæŌö¼Ó0.54 g£¬ŌņøĆÓŠ»śĪļµÄ×ī¼ņŹ½ĪŖ_______________”£
(6)ŅŖČ·¶ØøĆÓŠ»śĪļµÄ·Ö×ÓŹ½£¬»¹ŅŖ_________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com