
��13�֣�
��1��ij��ѧʵ��С����г�������һƿʳ�ð״ף���CH3COOH������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����Ũ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9 ���±���4�ֳ���ָʾ���ı�ɫ��Χ��
|
ָʾ�� |
ʯ�� |
���� |
���� |
��̪ |
|
��ɫ��Χ��pH�� |
5.0��8.0 |
3.1��4.4 |
4.4��6.2 |
8.2��10.0 |
�ٸ�ʵ��Ӧѡ�� ��ָʾ����
����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ mL��

��Ϊ��Сʵ������ͬѧһ������������ʵ�飬����ÿ
����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ��
�����¼���£�

���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ������� ��
A��ʵ�����ʱ�����ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ��������첿�ֳ�����Һ
C��ʢװ�״���Һ�ĵζ���������ˮϴ����δ�ð״���Һ��ϴ
D���μ�NaOH��Һʱ��δ������տ�����Һ��ɫ������ֹͣ�ζ�
��2���Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ���������ϵ�֪������������ˮ���л�ԭ�ԣ�����������KMnO4��Һ���еζ���
2MnO4����5H2C2O4��6H�� 2Mn2����10CO2����8H2O
����ͬѧ����˵ζ��ķ����ⶨxֵ��
��ȡ1.260 g�����ᾧ�壬���Ƴ�100.00 mLˮ��ҺΪ����Һ��
ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4��
��Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
��ش�
�ٵζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�\

�� ��ʵ��ζ��ﵽ�յ�ı�־��
��ͨ���������ݣ������x=
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
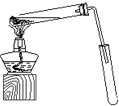 ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�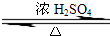 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O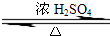 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
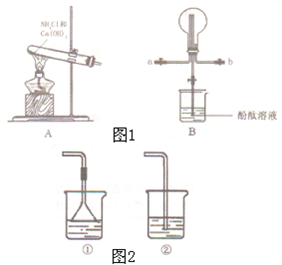 ij��ѧʵ��С��ͬѧ������ͼ1װ���Ʊ���������̽�����������ʣ�������������ȥ����
ij��ѧʵ��С��ͬѧ������ͼ1װ���Ʊ���������̽�����������ʣ�������������ȥ����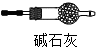 �ң�
�ң�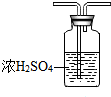 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�갲��ʡ�Ϸ�һ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣���1��ij��ѧС���ͬѧ��ѧϰ��NaHCO3��Na2CO3���й�֪ʶ��������ʵ�飺����֧�Թ��зֱ����3mL4 mol��L��1ϡ���ᣬ��������װ��0.3g NaHCO3�� Na2CO3��ĩ��С����ֱ�������֧�Թܿڡ��������ڵ�NaHCO3��Na2CO3ͬʱ�����Թ��У��۲쵽�������£�
���Թ��У���������������弰��Ӧ���ʵ��������___________ _��
��ʢ______________���Թ��������ø���С�������֮��ԼΪ����������ȣ�____________��
�ۼ�ͬѧ���ִ����Թܣ�����ʢNaHCO3��ĩ���Թܱ��䣬��ʢNa2CO3
���Թ��¶������ߡ��ɴ����ó���������״̬��Σ�NaHCO3��HCl��ӦΪ���ȷ�Ӧ����Na2CO3��HCl��ӦΪ���ȷ�Ӧ���������к��ȣ���ͬѧд���������Ȼ�ѧ����ʽ��
HCO3��(aq)��H+(aq)��H2O(l)��CO2(g)�� ��H>0
CO32��(aq)��2H+(aq)��H2O(l)��CO2(g)�� ��H<0
���½��۵ķ����Ƿ���ȷ____________�����ȷ������ȷ����
��2��Ϊ�о������Ȼ��Ƿ��ȷ�Ӧ����������������ʵ�飨ÿ��ʵ�����3��ƽ��ʵ�飬ȡƽ��ֵ����
| ��� | �Լ�1 | �Լ�2 | ���ǰ�¶� | ��Ϻ��������¶� |
| �� | 35mLˮ | 2.5g NaHCO3���� | 20�� | 18.5�� |
| �� | 35mLˮ | 3.2g Na2CO3���� | 20�� | 24.3�� |
| �� | 35mL ϡ���� | ��2.5g NaHCO3�ı�����Һ32.5mL | 20�� | 19�� |
| �� | 35mL ϡ���� | ��3.2g Na2CO3�ı�����Һ23.1 mL+10mlˮ | 20�� | 24.2�� |
| �� | 35mL ϡ���� | 2.5gNaHCO3���� | 20�� | 16.2�� |
| �� | 35mL ϡ���� | 3.2g Na2CO3���� | 20�� | 25.1�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com