
̼��﮹㷺Ӧ�����մɺ�ҽҩ��������﮻�ʯ����Ҫ�ɷ�ΪLiAlSi2O6��Ϊԭ�����Ʊ�Li2CO3�Ĺ����������£�
��֪����2LiAlSi2O6��H2SO4(Ũ)  Li2SO4��Al2O3��4SiO2��H2O��
Li2SO4��Al2O3��4SiO2��H2O��
��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��PH�ֱ�Ϊ3.2��4.7��9.0��11.1
��ijЩ���ʵ��ܽ�ȣ�S�����ұ�
��ش��������⣺
��1��﮻�ʯ��Ũ�����ȡ֮ǰҪ�����ϸ������Ŀ���� ��
��2����Һa�к���Li+��SO42-,������Fe3+��Al3+��Fe2+��Mg2+ ��Ca2+��Na+�����ʣ���������ڽ����¼���ʯ��ʯ�Ե�����Һ��pH��6.0��6.5����ʱ���������������� ��
��3�����������Һa�м���ij��Ӽ�����Ϊ������H2O2��Һ��ʯ�����Na2CO3��Һ��������Ӧ�����ӷ���ʽ�� ��
��4��������м��뱥��Na2CO3��Һ���˺���Ҫ����ˮϴ�ӵ�ԭ���� ��
��5������Һc�пɻ��յ���Ҫ������ ��
��1������﮻�ʯ������ĽӴ�������ӿ������Ӧ���ʣ���߽����ʣ�2�֣�
��2��Al3+��Fe3+��2�֣� ��3��2Fe2+��H2O2��2H+��2Fe3+��2H2O��3Ca(OH)2��2Fe3+��3Ca2+��2Fe(OH)3����
Ca(OH)2��Mg2+��Ca2+��Mg(OH)2����Ca2+��CO32-��CaCO3�� ��4�֣�
��4��Li2CO3���ܽ�����¶����߶���С����ˮϴ�ӿɼ���Li2CO3����ʧ��2�֣� ��5��Na2SO4��2�֣�
���������������1������Ӧ��ĽӴ���������Լӿ췴Ӧ���ʡ����Խ�﮻�ʯ���ϸ������Ŀ��������﮻�ʯ������ĽӴ�������ӿ������Ӧ���ʣ���߽����ʡ�
��2������Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��4.7��9.0��11.1�������������ʯ��ʯ�Ե�����Һ��pH��6.0��6.5����ʱ����������������Fe3+��Al3+��
��3�������������ӵij���pH�ϴ����Ա������������������������������������ӣ��Լ����ڳ�ȥ��˫��ˮ����ǿ�����ԣ��������������ӣ���Ӧ�����ӷ���ʽΪ2Fe2+��H2O2��2H+��2Fe3+��2H2O��ʯ�����Na2CO3��Һ�ֱܷ��������ӡ�þ�����Լ������ӽ���γɳ�������ȥ����Ӧ�����ӷ���ʽ�ֱ���3Ca(OH)2��2Fe3+��3Ca2+��2Fe(OH)3����Ca(OH)2��Mg2+��Ca2+��Mg(OH)2��Ca2+��CO32-��CaCO3����
��4�������ܽ�ȱ���֪��̼��﮵��ܽ�����¶ȵ����߶����ͣ��������ˮϴ�ӿɼ���Li2CO3����ʧ��
��5������ת��ͼ��֪��������̼��﮵�ͬʱ���������������ɣ����Դ���Һc�пɻ��յ���Ҫ������Na2SO4��
���㣺����̼����Ʊ�����ͼ���й��жϡ������Լ�ʵ����������۵�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
п�̷ϵ�ؾ���������������������п��̼���̣�����������ҵ��̼���̹������£�
�Իش��������⣺
(1)����Ԫ�����ڱ���λ�ڵ�________���ڣ���________�塣
(2)����1�Ͳ���2�ǽ�MnO2ת��ΪMnO���������ᣬ���в���2�е�����������һ�����̲����ĸ���Ʒ����д�����������ķ�Ӧ����ʽ__________________________��
(3)����3�Ͳ���4���dz����ʡ�
��X��һ�֡���ɫ������������X��________(�ѧʽ)��
�ڲ���3�dz�ȥ����Fe2�����������ֺͱ�Ҫ�ķ���ʽ������ȥFe2���ķ���(��֪�������ӳ�����pH��ΧΪFe3����2.7��3.7��Mn2����8.6��10.1��Fe2����7.6��9.6)_________________________________________________________��
�۲���4����Ҫ��Ӧ����ʽΪ��MeSO4��BaS=MeS����BaSO4��(Me��ҪΪPb��Cd��Hg��)�������ȥ���ʵ�ԭ����_________________________________________________��
(4)��֪���в���5�IJ���ʱ����Һ3(��Ҫ�ɷ�ΪMnSO4)�����������ɫ��ζ�����ݣ�����5��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ĽṹΪCH3��CH2��COOH,�������ǰ�ȫ��Ч�ķ�ù��������,һ���Լ�ʽ̼��пΪԭ�ϵ�����������������: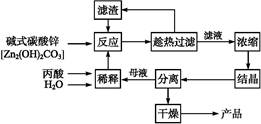
| ��� | n(����)�� n(��ʽ̼��п) | ��Ӧ�¶�/�� | ����п����/% |
| 1 | 1��0.25 | 60 | 67.2 |
| 2 | 1��0.25 | 80 | 83.5 |
| 3 | 1��0.25 | 100 | 81.4 |
| 4 | 1��0.31 | 60 | 89.2 |
| 5 | 1��0.31 | 80 | 90.1 |
| 6 | 1��0.31 | 100 | 88.8 |
 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ĽṹΪCH3��CH2��COOH,�������ǰ�ȫ��Ч�ķ�ù��������,һ���Լ�ʽ̼��пΪԭ�ϵ�����������������:
| ��� | n(����)�� n(��ʽ̼��п) | ��Ӧ�¶�/�� | ����п����/% |
| 1 | 1��0.25 | 60 | 67.2 |
| 2 | 1��0.25 | 80 | 83.5 |
| 3 | 1��0.25 | 100 | 81.4 |
| 4 | 1��0.31 | 60 | 89.2 |
| 5 | 1��0.31 | 80 | 90.1 |
| 6 | 1��0.31 | 100 | 88.8 |
 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����ѧʽ��ʾΪMgCO3��CaCO3��Ϊԭ���Ʊ�������þ�Ĺ����������£�

��1����������ͼ�жϰ���ʯ�����ա������������Ҫ�ɷ� _____ �������ա��¶�Ӧ������ _____ ��
��2������ͼ�С����ȷ�Ӧ���Ļ�ѧ����ʽΪ _ _________________________ ��
��3������������Һ��pH=9.5����ʱ��Һ��c(Mg2+)= _______ ����֪Ksp[Mg(OH)2]=5.61��10-12����
��4���ù����п���ѭ��ʹ�õ������� �� ���ѧʽ����
��5����ͳ���ս�����ʯ�ֽ�Ϊ����þ�������ƺ���ȡ���ù��ղ������հ���ʯ �ķ��������ŵ��� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ѹ㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
�������ѿ����������ַ����Ʊ���
����1��TiCl4ˮ������TiO2��xH2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2���˷����Ʊ��õ��������������ѡ�
��1���� TiCl4ˮ������TiO2��x H2O�Ļ�ѧ����ʽΪ_______________________________��
�� ����TiO2��x H2O��Cl���Ƿ����ķ�����______________________________��
����2�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�
��2��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ�� ��
��3������Һ�г���TiO2+֮����еĽ����������� ��
��4����Fe�������� ��
��.�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н��У������ԭ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ɸ���о��ȵ��ṹ������ɸɸ�����ü���ͼ�����ڷ���ɸ�������������ߣ����ȶ���ǿ��������������û�е��ŵ㣬ʹ�÷���ɸ��ù㷺��Ӧ�á�ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�

�ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɣ�����������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10����
��1������ɸ�Ŀ�ֱ��Ϊ4A(1 A=10-10m)��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч����������(����ֱ��Ϊ4.65A)���춡��(����ֱ��Ϊ5.6A)Ӧ��ѡ�� �ͷ���ɸ��
��2��A12(SO4)3��Һ��Na2SiO3��Һ��Ӧ���ɽ�������ӷ���ʽΪ
��3��������������������Һ�ﺬ�е����ӳ�H+��OH-�⣬��ҪΪ ���������н��������ӵIJ���������
��4����NH3��H2O����pH���ȵ�90�沢���ȹ��˵�ԭ�������
��5�����������������÷���ɸ�Ļ�ѧʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������LiFePO4��һ��������������ӵ�صĵ缫���ϡ�ij�����������졢﮻�ʯLiAl��SiO3��2��������Ca2+��Mg2+���Σ���̼�۵�ԭ����������������ﮡ�����Ҫ�����������£�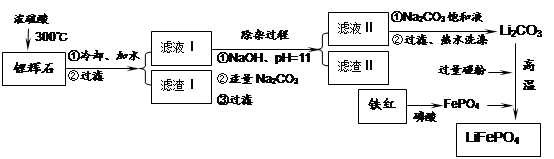
��֪��2LiAl��SiO3��2 + H2SO4(Ũ)  Li2SO4 + Al2O3��4SiO2��H2O��
Li2SO4 + Al2O3��4SiO2��H2O��
| �¶�/�� | 20 | 40 | 60 | 80 |
| �ܽ��(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
| �ܽ��(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |
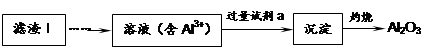
 LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ��
LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
(1)1791�꣬����ҽ��·�������Ȼ���Ƽ�ר������ʳ�Ρ�Ũ���ᡢʯ��ʯ��úΪԭ���Ƽ�÷�����������
����NaCl��H2SO4��Ӧ��Na2SO4��2NaCl��H2SO4 Na2SO4��2HCl��
Na2SO4��2HCl��
���ý�̿��ԭNa2SO4��Na2S��Na2SO4��4C Na2S��4CO��
Na2S��4CO��
����������ʯ��ʯ��Ӧ��Na2CO3��Na2S��CaCO3 Na2CO3��CaS
Na2CO3��CaS
(2)����˵����ȷ���� (����)��
| A���ڢ���������������ԭ��Ӧ |
| B��ֻ�еڢڲ���������ԭ��Ӧ |
| C���÷����������豸����û�и�ʴ |
| D���÷����Ի�����Ⱦ��С |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com