
���� ��1����һ�ݼ��뼸��AgNO3��Һ���г�����������Һ�п��ܺ���Cl-��CO32-��SO42-��
��2���ڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ��������ǰ�����һ����笠����ӣ�笠����ӵ����ʵ�����0.02mol���������ɣ�һ������Fe3+��Mg2+��
��3���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��˵��ԭ��Һ��һ������Al3+��������ӹ����֪һ��������CO32-��1.02gΪ�������������������ʵ���Ϊ��$\frac{1.02g}{102g/mol}$=0.01mol��˵��ÿ����Һ�к���0.02molAl3+��
��4�������ݼ�����BaCl2��Һ�ð�ɫ�������ð�ɫ����Ϊ���ᱵ��˵��ԭ��Һ��һ������SO42-����һ�������ڱ����ӣ���������������ϴ�ӡ����������Ϊ11.65g�������ᱵ�����ʵ���Ϊ��$\frac{11.65g}{233g/mol}$=0.05mol��˵��ÿ����Һ�к���0.05molSO42-��
0.02molAl3+���������Ϊ0.06mol��0.02mol笠����Ӵ���0.02mol����ɣ���0.05molSO42-���������Ϊ0.1mol�����ݵ���غ��֪��˵����Һ��һ��������������K+���ݴ˶Ը�ѡ������жϣ�
��� �⣺��һ�ݼ��뼸��AgNO3��Һ���г�����������Һ�п��ܺ���Cl-��CO32-��SO42-��
�ڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ��������ǰ�����һ����笠����ӣ�笠����ӵ����ʵ�����0.02mol���������ɣ�һ������Fe3+��Mg2+���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��˵��ԭ��Һ��һ������Al3+��������ӹ����֪һ��������CO32-��1.02gΪ�������������������ʵ���Ϊ��$\frac{1.02g}{102g/mol}$=0.01mol��˵��ÿ����Һ�к���0.02molAl3+��
�����ݼ�����BaCl2��Һ�ð�ɫ�������ð�ɫ����Ϊ���ᱵ��˵��ԭ��Һ��һ������SO42-����һ�������ڱ����ӣ���������������ϴ�ӡ����������Ϊ11.65g�������ᱵ�����ʵ���Ϊ��$\frac{11.65g}{233g/mol}$=0.05mol��˵��ÿ����Һ�к���0.05molSO42-��
0.02molAl3+���������Ϊ0.06mol��0.02mol笠����Ӵ���0.02mol����ɣ���0.05molSO42-���������Ϊ0.1mol�����ݵ���غ��֪��˵����Һ��һ��������������K+��
��1�����ݷ�����֪��һ�����ڵ�����Ϊ��K+��NH4+��Al3+��SO42-��һ�������ڵ�����Ϊ��Fe3+��Mg2+��CO32-��Ba2+�����ݷ�����֪����ȷ���Ƿ����Cl-��������Һ�п��ܴ���Cl-��
�ʴ�Ϊ��Fe3+��Mg2+��CO32-��Ba2+��Cl-��
��2��笠����ӵ����ʵ�����0.02mol������0.02molAl3+������0.05molSO42-��0.02molAl3+���������Ϊ0.06mol��0.02mol笠����Ӵ���0.02mol����ɣ���0.05molSO42-���������Ϊ0.1mol�����ݵ���غ��֪��˵����Һ��һ��������������K+����Һ���Ϊ0.1L��������Ũ�ȷֱ�Ϊ0.2mol/L��0.2mol/L��0.5mol/L��
�ʴ�Ϊ��NH4+��0.2mol/L��Al3+��0.2mol/L��SO42-��0.5mol/L��
��3����Һ������ɵ������ʵ���Ϊ��n��NH4+��+3n��Al3+��=0.02mol+3��0.02mol=0.08mol��
����ɵ������ʵ���Ϊ��2n��SO42-��=0.1mol�����ݵ���غ㣬��Һ��һ�����ڼ����ӣ����ڿ��ܴ��������ӣ�������ӵ����ʵ�������Ϊ��0.1mol-0.08mol=0.02mol��c��K+����0.2mol��l-1���ʴ�Ϊ���ǣ����ݵ���غ㣬��Һ��һ�����ڼ����ӣ�
���� ���⿼�鳣�����ӵļ��飬��Ŀ�Ѷ��еȣ����ö���ʵ��Ͷ�������������ϵ�ģʽ�������˽����Ѷȣ�ͬʱ�漰���ӹ��桢���ӷ�Ӧ�ȶ��ǽ�����ע�����Ϣ��������K+��ȷ���׳���ʧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�1.12LSO3������ԭ������0.2NA | |
| B�� | 3.9gNa2O2����������CO2ʱת�Ƶ�������0.05NA | |
| C�� | 20g��ˮ�к���������Ϊ8NA | |
| D�� | 28g��ϩ�ͻ����飨C4H8���Ļ�������к��е�̼ԭ����Ϊ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
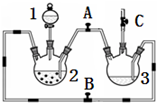 ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��
ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼��ĵ���Ϊ���ȹ��̣�����̼���������ᷴӦ���� | |
| B�� | ��֪NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-57.3kJ•mol-1���� 40.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�����������57.3 kJ | |
| C�� | ͨ��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-57.3kJ•mol-1����֪ 1molH2O��l����ȫ������Ҫ����57.3kJ | |
| D�� | ���ȷ�ӦΪ���ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com