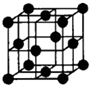
A��B��C��D��E�Ƕ������е�5��Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С����AΪ��Ԫ�أ�Bԭ�ӵ��������������ڲ��������2������B��2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�DԪ����AԪ��ͬ���壬ԭ����������̼Ԫ�أ���DΪ��Ԫ�أ�E�ĵ���Ϊ��ɫ���壬�����ڶ���̼����EΪ��Ԫ�أ�EԪ����CԪ��ͬ���壬��CΪ��Ԫ�أ�
��AΪ��Ԫ�أ�A��������ΪH
-��H
-�Ľṹʾ��ͼΪ
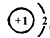
���ʴ�Ϊ��
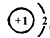
��
������������ȼ�����ɹ������ƣ���Ӧ����ʽΪ2Na+O
2Na
2O
2���������Ƽ�����з�̪��ˮ�У�����������ˮ��Ӧ�����������ƺ�����������ɫ����ų�����Һ��죬�������ƾ���ǿ�����ԣ���ɫ��ȥ��
�ʴ�Ϊ��2Na+O
2Na
2O
2������ɫ����ų�����Һ�ȱ�죬����ɫ��
�۽�9g̼������������������ȼ�գ����ɶ�����̼
=0.75mol���������������̼ͨ��1L1.0mol?L
-1NaOH��Һ�У�������̼�������������ʵ���֮��Ϊ0.75��1����������̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊx��y����x+y=0.75mol��2x+y=1mol�����x=0.25mol��y=0.5mol�����ڳʼ���c��OH
-����c��H
+����̼�����̼�������ˮ��̶ȶ���������c��HCO
3-����c��CO
32-��������Һ������Ũ���ɴ�С������˳����c��Na
+����c��HCO
3-����c��CO
32-����c��OH
-����c��H
+����
�ʴ�Ϊ��c��Na
+����c��HCO
3-����c��CO
32-����c��OH
-����c��H
+����
�ܽ�E�ĵ�����������C�ĵ�����ȼ�գ�������Ҫ����ΪY��YΪSO
2��XΪNa
2O
2����SO
2ͨ��Na
2O
2�У����ܷ����ķ�Ӧ�Ļ�ѧ����ʽ��2Na
2O
2+2SO
2�T2Na
2SO
3+O
2����Na
2O
2+SO
2�TNa
2SO
4��
�ʴ�Ϊ��2Na
2O
2+2SO
2�T2Na
2SO
3+O
2����Na
2O
2+SO
2�TNa
2SO
4��

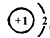





 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�