
ЎҫМвДҝЎҝУР»ъ»ҜәПОпGКЗәПіЙО¬ЙъЛШАаТ©ОпөДЦРјдМеЈ¬ЖдәПіЙВ·ПЯИзПВЈә

ЖдЦРAЎ«F·ЦұрҙъұнТ»ЦЦУР»ъ»ҜәПОпЈ¬әПіЙВ·ПЯЦРІҝ·ЦІъОпј°·ҙУҰМхјюТСВФИҘТСЦӘЈә

GОӘ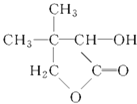 Ј»
Ј»
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©GөД·ЦЧУКҪ_____________Ј»DЦР№ЩДЬНЕөДГыіЖКЗ_________ЎЈ
ЈЁ2Ј©өЪўЪІҪ·ҙУҰөД»ҜС§·ҪіМКҪОӘ__________________________ЎЈ
ЈЁ3Ј©өЪўЫІҪ·ҙУҰөД»ҜС§·ҪіМКҪОӘ__________________________ЎЈ
ЈЁ4Ј©РҙіцFөДҪб№№јтКҪ_____________ЎЈ
ЈЁ5Ј©өЪўЩ~ўЮІҪ·ҙУҰЦРКфУЪјУіЙ·ҙУҰөДУР___________________Ј»КфУЪИЎҙъ·ҙУҰөДУР
__________________________ЎЈЈЁМоІҪЦиұаәЕЈ©
ЈЁ6Ј©Н¬КұВъЧгПВБРМхјюөДEөДН¬·ЦТм№№МеУР_____________ЦЦЎЈ
ўЩЦ»ә¬Т»ЦЦ№ЩДЬНЕЈ»
ўЪБҙЧҙҪб№№ЗТОЮЎӘOЎӘOЎӘЈ»
ўЫәЛҙЕ№ІХсЗвЖЧЦ»УР2Чй·еЎЈ
Ўҫҙр°ёЎҝЈЁ1Ј©C6H10O3хҘ»щЎўИ©»щЎўфЗ»щ¶аРҙЎўВ©РҙЎўРҙҙнҫщІ»өГ·Ц
ЈЁ2Ј©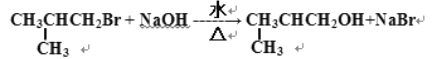
ЈЁ3Ј©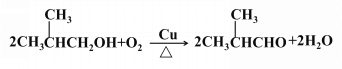
ЈЁ4Ј©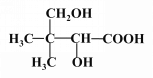
ЈЁ5Ј©ўЩўЬўЮЎўўЪўЭ ЈЁВ©РҙөГТ»·ЦЈ¬РҙҙнІ»өГ·ЦЈ© ЈЁ6Ј©3
ЎҫҪвОцЎҝ
КФМв·ЦОцЈәТм¶ЎП©әНде»ҜЗв·ўЙъјУіЙ·ҙУҰЙъіЙдеҙъМюAЈ¬AәНЗвСх»ҜДЖөДЛ®ИЬТә·ўЙъИЎҙъ·ҙУҰЙъіЙҙјBЈ¬Bұ»СхЖшСх»ҜЙъіЙТм¶ЎИ©Ј¬ФтBКЗ2-јЧ»щ-1-ұыҙјЈ¬AКЗ2-јЧ»щ-1-деұыНйЈ¬Тм¶ЎИ©әНC·ҙУҰЙъіЙDЈ¬DЛ®ҪвЙъіЙТТҙјәНEЈ¬ёщҫЭМвёшРЕПўЦӘЈ¬EәНЗвЖш·ўЙъјУіЙ·ҙУҰЙъіЙFЈ¬FјУИИ·ЦҪвЙъіЙЛ®әНGЈ¬ёщҫЭGөДҪб№№јтКҪЦӘЈ¬FөДҪб№№јтКҪОӘЈәHOCH2 CЈЁCH3Ј©2CHOHCOOHЈ¬EөДҪб№№јтКҪОӘЈәOHCCЈЁCH3Ј©2CHOHCOOHЈ¬DөДҪб№№јтКҪОӘЈәOHCCЈЁCH3Ј©2CHOHCOOCH2CH3Ј¬CөДҪб№№јтКҪОӘЈәOHCCOOCH2CH3Ј¬
ЈЁ1Ј©ёщҫЭGөДҪб№№јтКҪЦӘЈ¬GөД·ЦЧУКҪОӘC6H10O3Ј¬DөДҪб№№јтКҪОӘOHCCЈЁCH3Ј©2CHOHCOOCH2CH3Ј¬ә¬УРөД№ЩДЬНЕКЗхҘ»щЎўИ©»щЎўфЗ»щЈ»
ЈЁ2Ј©өЪўЪІҪВұҙъМю·ўЙъөДЛ®Ҫв·ҙУҰөД»ҜС§·ҪіМКҪОӘ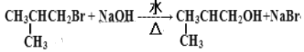 Ј»
Ј»
ЈЁ3Ј©өЪўЫІҪ·ҙУҰөД»ҜС§·ҪіМКҪОӘ![]() Ј»
Ј»
ЈЁ4Ј©РҙіцFөДҪб№№јтКҪЈәHOCH2 CЈЁCH3Ј©2CHOHCOOHЎЈ
ЈЁ5Ј©өЪўЩ~ўЮІҪ·ҙУҰЦРўЩКЗјУіЙ·ҙУҰЈ¬ўЪКЗИЎҙъ·ҙУҰЈ¬ўЫКЗСх»Ҝ·ҙУҰЈ¬ўЬјУіЙ·ҙУҰЈ¬ўЭИЎҙъ·ҙУҰЈ¬ўЮјУіЙ·ҙУҰЈ¬ЛщТФКфУЪјУіЙ·ҙУҰөДУРўЩўЬўЮЈ¬ИЎҙъ·ҙУҰөДУРўЪўЭЈ»
ЈЁ6Ј©Н¬КұВъЧгМхјюөДEөДН¬·ЦТм№№МеУРЈәCH3COOCH2CH2OOCCH3ЎўCH3CH2OOCCOOCH2CH3ЎўCH3OOCCH2CH2COOCH3 Ј¬№І3ЦЦЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝЈЁ1Ј©УРПВБРјёЧйОпЦКЈ¬ЗлҪ«РтәЕМоИлПВБРҝХёсДЪЈә
AЎўCH2=CH-COOHәНУНЛбЈЁC17H33COOHЈ© BЎў12C60әНКҜД«
CЎў![]() әН
әН![]() DЎў35ClәН37Cl EЎўТТҙјәНТТ¶юҙј
DЎў35ClәН37Cl EЎўТТҙјәНТТ¶юҙј
ўЩ»ҘОӘН¬О»ЛШөДКЗ Ј»
ўЪ»ҘОӘН¬ПөОпөДКЗ Ј»
ўЫ»ҘОӘН¬ЛШТмРОМеөДКЗ Ј»
ўЬ»ҘОӘН¬·ЦТм№№МеөДКЗ Ј»
ўЭјИІ»КЗН¬ПөОпЈ¬УЦІ»КЗН¬·ЦТм№№МеЈ¬ТІІ»КЗН¬ЛШТмРОМеЈ¬ө«ҝЙҝҙіЙКЗН¬Т»АаОпЦКөДКЗ ЎЈ
ЈЁ2Ј©ЗлРҙіцПВБР·ҙУҰөД»ҜС§·ҪіМКҪЈә
ўЩУЙұыП©ЦЖИЎҫЫұыП©Јә Ј»Ј»
ўЪұы°ұЛбЛхҫЫРОіЙ¶алДЈә Ј»
ўЫөн·ЫЛ®ҪвЈә Ј»
ўЬұыИ©УлРВЦЖөДЗвСх»ҜНӯРьЧЗТә·ҙУҰЈә Ј»
ЈЁ3Ј©УГТ»ЦЦКФјБҪ«ПВБРёчЧйОпЦКјшұрҝӘЈ¬РҙіцЖдГыіЖЎЈ
ўЩ![]() әН
әН![]() Јә Ј»
Јә Ј»
ўЪ ![]() Ј¬
Ј¬![]() әНC6H12(јәП©)Јә Ј»
әНC6H12(јәП©)Јә Ј»
ўЫ ![]() Ј¬CCl4әНТТҙј ЎЈ
Ј¬CCl4әНТТҙј ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝұҪјЧЛбДЖЈЁ![]() Ј¬ЛхРҙОӘNaAЈ©ҝЙУГЧчТыБПөД·АёҜјБЎЈСРҫҝұнГчұҪјЧЛбЈЁHAЈ©өДТЦҫъДЬБҰПФЦшёЯУЪAЁCЎЈТСЦӘ25 ЎжКұЈ¬HAөДKa=6.25ЎБ10ЁC5Ј¬H2CO3өДKa1=4.17ЎБ10ЁC7Ј¬Ka2=4.90ЎБ10ЁC11ЎЈФЪЙъІъМјЛбТыБПөД№эіМЦРЈ¬іэБЛМнјУNaAНвЈ¬»№РијУС№ідИлCO2ЖшМеЎЈПВБРЛө·ЁХэИ·өДКЗЈЁОВ¶ИОӘ25 ЎжЈ¬І»ҝјВЗТыБПЦРЖдЛыіЙ·ЦЈ©ЈЁ Ј©
Ј¬ЛхРҙОӘNaAЈ©ҝЙУГЧчТыБПөД·АёҜјБЎЈСРҫҝұнГчұҪјЧЛбЈЁHAЈ©өДТЦҫъДЬБҰПФЦшёЯУЪAЁCЎЈТСЦӘ25 ЎжКұЈ¬HAөДKa=6.25ЎБ10ЁC5Ј¬H2CO3өДKa1=4.17ЎБ10ЁC7Ј¬Ka2=4.90ЎБ10ЁC11ЎЈФЪЙъІъМјЛбТыБПөД№эіМЦРЈ¬іэБЛМнјУNaAНвЈ¬»№РијУС№ідИлCO2ЖшМеЎЈПВБРЛө·ЁХэИ·өДКЗЈЁОВ¶ИОӘ25 ЎжЈ¬І»ҝјВЗТыБПЦРЖдЛыіЙ·ЦЈ©ЈЁ Ј©
AЈ®ПаұИУЪОҙідCO2өДТыБПЈ¬МјЛбТыБПөДТЦҫъДЬБҰҪПөН
BЈ®МбёЯCO2ідЖшС№БҰЈ¬ТыБПЦРc(AЁC)І»ұд
CЈ®өұpHОӘ5.0КұЈ¬ТыБПЦР![]() =0.16
=0.16
DЈ®МјЛбТыБПЦРёчЦЦБЈЧУөДЕЁ¶И№ШПөОӘЈәc(H+)=c(![]() )+c(
)+c(![]() )+c(OHЁC)ЁCc(HA)
)+c(OHЁC)ЁCc(HA)
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРУР№ШОпЦКөДРФЦКУлУҰУГПа¶ФУҰөДКЗ
AЈ®SO2ҫЯУРСх»ҜРФЈ¬ҝЙУГУЪЖҜ°ЧЦҪҪ¬
BЈ®Зв·ъЛбҫЯУРЛбРФЈ¬ҝЙУГУЪөсҝМІЈБ§
CЈ®¶юСх»ҜВИҫЯУР»№ФӯРФЈ¬ҝЙУГУЪЧФАҙЛ®өДЙұҫъПы¶ҫ
DЈ®NH3ҫЯУР»№ФӯРФЈ¬ҝЙУГNH3УлЧЖИИCuOЧчУГЦЖИЎЙЩБҝN2
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПтДіГЬұХИЭЖчЦРідИл1 mol COәН2 mol H2O(g)Ј¬·ўЙъ·ҙУҰЈәCOЈ«H2O(g)![]() CO2Ј«H2ЎЈөұ·ҙУҰҙпөҪЖҪәвКұЈ¬COөДМе»э·ЦКэОӘxЎЈИфО¬іЦИЭЖчөДМе»эәНОВ¶ИІ»ұдЈ¬ЖрКјОпЦК°ҙПВБРЛДЦЦЕдұИідИлёГИЭЖчЦРЈ¬ҙпөҪЖҪәвКұCOөДМе»э·ЦКэҙуУЪxөДКЗ(ЎЎЎЎ)
CO2Ј«H2ЎЈөұ·ҙУҰҙпөҪЖҪәвКұЈ¬COөДМе»э·ЦКэОӘxЎЈИфО¬іЦИЭЖчөДМе»эәНОВ¶ИІ»ұдЈ¬ЖрКјОпЦК°ҙПВБРЛДЦЦЕдұИідИлёГИЭЖчЦРЈ¬ҙпөҪЖҪәвКұCOөДМе»э·ЦКэҙуУЪxөДКЗ(ЎЎЎЎ)
A. 0.5 mol COЈ«2 mol H2O(g)Ј«1 mol CO2Ј«1 mol H2
B. 1 mol COЈ«1 mol H2O(g)Ј«1 mol CO2Ј«1 mol H2
C. 0.5 mol COЈ«1.5 mol H2O(g)Ј«0.4 mol CO2Ј«0.4 mol H2
D. 0.5 mol COЈ«1.5 mol H2O(g)Ј«0.5 mol CO2Ј«0.5 mol H2
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТСЦӘAЎўBЎўCЎўDКЗФӯЧУРтКэТАҙОФцҙуөДЛДЦЦ¶МЦЬЖЪЦчЧеФӘЛШЈ¬AөДЦЬЖЪКэөИУЪЖдЦчЧеРтКэЈ¬BФӯЧУөДјЫөзЧУЕЕІјОӘnsnnpnЈ¬DКЗөШҝЗЦРә¬БҝЧо¶аөДФӘЛШЎЈEКЗөЪЛДЦЬЖЪpЗшөДФӘЛШЗТЧоНвІгЦ»УР2¶ФіЙ¶ФөзЧУЈ¬FКЗ29әЕФӘЛШЎЈ
ЈЁ1Ј©BЎўCЎўDИэФӘЛШөЪТ»өзАлДЬУЙҙуөҪРЎөДЛіРтОӘ ЈЁУГФӘЛШ·ыәЕұнКҫЈ©
ЈЁ2Ј©BD32-ЦРРДФӯЧУФУ»Ҝ№мөАөДАаРНОӘ________ФУ»ҜЈ»CA4+өДҝХјд№№РНОӘ______________ЎЈ
ЈЁ3Ј©»щМ¬EФӯЧУөДјЫөзЧУЕЕІјНј______________________________ЎЈ
ЈЁ4Ј©1mol BCЈӯЦРә¬УРҰРјьөДКэДҝОӘ______________ЎЈ
ЈЁ5Ј©ұИҪПDЎўEФӘЛШЧојтөҘЗв»ҜОпөД·РөгёЯөНЈә ЈЁУГ»ҜС§КҪұнКҫЈ©ЎЈ
ЈЁ6Ј©CЎўFБҪФӘЛШРОіЙөДДі»ҜәПОпөДҫ§°ыҪб№№ИзНјЛщКҫЈ¬¶ҘөгОӘCФӯЧУЎЈФтёГ»ҜәПОпөД»ҜС§КҪКЗ Ј¬CФӯЧУөДЕдО»КэКЗ ЎЈИфПаБЪCФӯЧУәНFФӯЧУјдөДҫаАлОӘa cmЈ¬°ў·ьЩӨөВВЮіЈКэОӘNAЈ¬ФтёГҫ§МеөДГЬ¶ИОӘ________________gЈҜcm3ЈЁУГә¬aЎўNAөД·ыәЕұнКҫЈ©ЎЈ

Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝAЎўBЎўCЎўDОӘФӯЧУРтКэТАҙОФцҙуөДЛДЦЦФӘЛШЈ¬A2ЈӯәНB+ҫЯУРПаН¬өДөзЧУ№№РНЈ»CЎў DОӘН¬ЦЬЖЪФӘЛчЈ¬CәЛНвөзЧУЧЬКэКЗЧоНвІгөзЧУКэөД3ұ¶Ј»DФӘЛШЧоНвІгУРТ»ёцОҙіЙ¶ФөзЧУЎЈ»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ЛДЦЦФӘЛШЦРөзёәРФЧоРЎөДКЗ________ЈЁМоФӘЛШ·ыәЕЈ©Ј¬ЖдЦРCФӯЧУөДНвО§өзЧУЕЕІјНјОӘ________ЎЈ
ЈЁ2Ј©AәНBөДЗв»ҜОпЛщКфөДҫ§МеАаРН·ЦұрОӘ_________әН_________ЎЈ
ЈЁ3Ј©BЎўCҫщҝЙТФУлDРОіЙ»ҜәПОпЈ¬ЖдЦРИЫөгҪПёЯөДКЗ____ЈЁУГ»ҜС§КҪұнКҫЈ©
ЈЁ4Ј©AәНBҝЙРОіЙ1:1РНөД»ҜәПОпEЈ¬EөДөзЧУКҪОӘ_____
ЈЁ5Ј©»ҜәПОпD2AөДБўМе№№РНОӘ_________Ј¬ЦРРДФӯЧУөД№ВөзЧУ¶ФКэОӘ_________Ј¬өҘЦКDУлКӘИуөДNa2CO3·ҙУҰҝЙЦЖұёD2AЈ¬Жд»ҜС§·ҪіМКҪОӘ_______________ЎЈ
ЈЁ6Ј©AәНBДЬ№»РОіЙ»ҜәПОпFЈ¬Ждҫ§°ыҪб№№ИзНјЛщКҫЈ¬ҫ§°ыұЯіӨОӘ0.566nmЈ¬ F өД»ҜС§КҪОӘ______Ј»ҫ§°ыЦРA ФӯЧУөДЕдО»КэОӘ______Ј»ҫ§МеFөДГЬ¶И=______gЈ®cmЈӯ3ЈЁЦ»БРКҪЈ¬І»јЖЛгЈ©

Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ°лЛ®ГәЖшКЗ№ӨТөәПіЙ°ұөДФӯБПЖшЈ¬ЖдЦчТӘіЙ·ЦКЗH2ЎўCOЎўCO2ЎўN2әНH2OЈЁgЈ©ЎЈ°лЛ®ГәЖшҫӯ№эПВБРІҪЦиЧӘ»ҜОӘәПіЙ°ұөДФӯБПЎЈ

НкіЙПВБРМоҝХЈә
ЈЁ1Ј©°лЛ®ГәЖшә¬УРЙЩБҝБт»ҜЗвЎЈҪ«°лЛ®ГәЖшСщЖ·НЁИл____ИЬТәЦРЈЁМоРҙКФјБГыіЖЈ©Ј¬іцПЦ_______Ј¬ҝЙТФЦӨГчУРБт»ҜЗвҙжФЪЎЈ
ЈЁ2Ј©°лЛ®ГәЖшФЪНӯҙЯ»ҜПВКөПЦCOұд»»ЈәCO+H2O![]() CO2+H2
CO2+H2
Иф°лЛ®ГәЖшЦРVЈЁH2Ј©:VЈЁCOЈ©:VЈЁN2Ј©=38Јә28Јә22Ј¬ҫӯCOұд»»әуөДЖшМеЦРЈәVЈЁH2Ј©:VЈЁN2Ј©=____________ЎЈ
ЈЁ3Ј©јоТәОьКХ·ЁКЗНСіэ¶юСх»ҜМјөД·Ҫ·ЁЦ®Т»ЎЈТСЦӘЈә
Na2CO3 | K2CO3 | |
20ЎжјоТәЧоёЯЕЁ¶ИЈЁmol/LЈ© | 2.0 | 8.0 |
јоөДјЫёсЈЁФӘ/kgЈ© | 1.25 | 9.80 |
ИфСЎФсNa2CO3јоТәЧчОьКХТәЈ¬ЖдУЕөгКЗ__________Ј»ИұөгКЗ____________ЎЈИз№ыСЎФсK2CO3јоТәЧчОьКХТәЈ¬УГКІГҙ·Ҫ·ЁҝЙТФҪөөНіЙұҫЈҝ
___________________________________________
РҙіцХвЦЦ·Ҫ·ЁЙжј°өД»ҜС§·ҙУҰ·ҪіМКҪЎЈ_______________________
ЈЁ4Ј©ТФПВКЗІв¶Ё°лЛ®ГәЖшЦРH2ТФј°COөДМе»э·ЦКэөДКөСй·Ҫ°ёЎЈ
ИЎТ»¶ЁМе»эЈЁұкЧјЧҙҝцЈ©өД°лЛ®ГәЖшЈ¬ҫӯ№эПВБРКөСйІҪЦиІв¶ЁЖдЦРH2ТФј°COөДМе»э·ЦКэЎЈ

ўЩСЎУГәПККөДОЮ»ъКФјБ·ЦұрМоИлўсЎўўсЎўўфЎўўх·ҪҝтЦРЎЈ
ўЪёГКөСй·Ҫ°ёЦРЈ¬ІҪЦиўсЎўўтөДДҝөДКЗЈә ЎЈ
ўЫёГКөСй·Ҫ°ёЦРЈ¬ІҪЦи________ЈЁСЎМоЎ°ўфЎұ»тЎ°ўхЎұЈ©ҝЙТФИ·¶Ё°лЛ®ГәЖшЦРH2өДМе»э·ЦКэЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝЈЁ1Ј©ИзНјЛщКҫЧ°ЦГЈ¬ЧЬ·ҙУҰөДАлЧУ·ҪіМКҪОӘ_________________________ЎЈ

ЈЁ2Ј©ЦКБҝПаөИөДБҪөзј«·ҙУҰәуЦКБҝПаІо12 gЈ¬ФтөјПЯЦРНЁ№эБЛ____________mol өзЧУЎЈ
ЈЁ3Ј©ЖдЛыМхјюІ»ұдЈ¬ИфҪ«CuCl2ИЬТә»»ОӘNH4ClИЬТәЈ¬КҜД«өзј«·ҙУҰКҪ_____________________Ј¬ХвКЗУЙУЪNH4ClИЬТәПФ__________ (МоЎ°ЛбРФЎұЎ°јоРФЎұ»тЎ°ЦРРФЎұ)Ј¬УГАлЧУ·ҪіМКҪұнКҫИЬТәПФҙЛРФөДФӯТт____________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com