

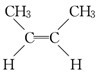
 ��˳ʽ2-��ϩ����
��˳ʽ2-��ϩ����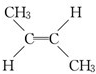 ����ʽ2-��ϩ����
����ʽ2-��ϩ����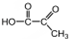 ��B����������H2�ӳɵõ�2-�����飬��д��B�Ľṹ��ʽCH3CH=CHC��CH3��=CHCH3��д��B�����Ӿ۷�Ӧ�ķ���ʽ
��B����������H2�ӳɵõ�2-�����飬��д��B�Ľṹ��ʽCH3CH=CHC��CH3��=CHCH3��д��B�����Ӿ۷�Ӧ�ķ���ʽ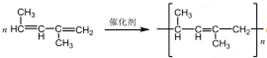 ��
������ ��1���������ж���̼̼��������ϩ����̼̼˫�����ݴ��жϣ�
��2������������Ϣ��֪��ijϩ��A��C4H8���������Ը��������Һ������ֻ�õ�����CH3COOH����AΪCH3CH=CHCH3������˳���칹����ص�д�ṹ��ʽ��
��3��������Ϣ��֪��CH2=CHC��CH3��=CH2������KMnO4��Һ��Ӧ���л�����̼̼˫����������̼��˫�����Ȼ���
��4�������л����CH3COOH�� �����������Ϣ�ɷ��Ƶ�B��Ӧ����CH3CH=���ֺ�=CHC��CH3��=���֣�B��������H2�ӳɵõ�2-�����飬��B�Ľṹ��ʽΪCH3CH=CHC��CH3��=CHCH3��B�����Ӿ۷�Ӧʱ������̼̼˫�������ѣ�����������1��4-�ӳɵķ�ʽ���мӾۣ���B������HBr�ļӳɣ�B��HBrΪ���ԳƷ��ӣ��ɷ���1��2-�ӳɣ������֣�1��4-�ӳ������֣�3��4-�ӳ������֣�1��2��3��4-�ӳ���6�֣�
�����������Ϣ�ɷ��Ƶ�B��Ӧ����CH3CH=���ֺ�=CHC��CH3��=���֣�B��������H2�ӳɵõ�2-�����飬��B�Ľṹ��ʽΪCH3CH=CHC��CH3��=CHCH3��B�����Ӿ۷�Ӧʱ������̼̼˫�������ѣ�����������1��4-�ӳɵķ�ʽ���мӾۣ���B������HBr�ļӳɣ�B��HBrΪ���ԳƷ��ӣ��ɷ���1��2-�ӳɣ������֣�1��4-�ӳ������֣�3��4-�ӳ������֣�1��2��3��4-�ӳ���6�֣�
��5������C������ʽC9H20������һ��ͬ���칹��C1��C2��C3ȴ�ֱ���ɶ���ֻ����1����Ӧ��ϩ����D1��D2��D3��������õ�����Aͬ���칹�������ڵ�����̼ԭ���Ϻ�����ԭ�ӵ�λ��ֻ��1�����������������A��ͬ���칹�壨C1��C2��C3��Ϊ��CH3��3C-C��CH3��2-CH2-CH3����CH3��2CH-C��CH3��2-CH��CH3��2��C��CH2-CH3��4�е�һ�֣�1molD3�������Ը��������Һ������ɲ���1molCO2��ͪ�����ʣ�����D3ΪCH3��3C-C��CH3��2-CH=CH2������C3ΪCH3��3C-C��CH3��2-CH2-CH3���ݴ˴��⣮
��� �⣺��1���������ж���̼̼��������ϩ����̼̼˫�������Լ������ߵ�ʵ�鷽��Ϊ��C3H6ͨ�����Ը��������Һ������Һ��ɫ����Ϊ��ϩ��������ɫ����Ϊ�����飬
�ʴ�Ϊ����C3H6ͨ�����Ը��������Һ������Һ��ɫ����Ϊ��ϩ��������ɫ����Ϊ�����飻
��2������������Ϣ��֪��ijϩ��A��C4H8���������Ը��������Һ������ֻ�õ�����CH3COOH����AΪCH3CH=CHCH3����˳���칹��Ϊ ��˳ʽ2-��ϩ����
��˳ʽ2-��ϩ���� ����ʽ2-��ϩ����
����ʽ2-��ϩ����
�ʴ�Ϊ�� ��˳ʽ2-��ϩ����
��˳ʽ2-��ϩ���� ����ʽ2-��ϩ����
����ʽ2-��ϩ����
��3��������Ϣ��֪��CH2=CHC��CH3��=CH2������KMnO4��Һ��Ӧ�л�����ṹ��ʽΪHOOCCOCH3��
�ʴ�Ϊ��HOOCCOCH3��
��4�������л����CH3COOH�� �����������Ϣ�ɷ��Ƶ�B��Ӧ����CH3CH=���ֺ�=CHC��CH3��=���֣�B��������H2�ӳɵõ�2-�����飬��B�Ľṹ��ʽΪCH3CH=CHC��CH3��=CHCH3��B�����Ӿ۷�Ӧʱ������̼̼˫�������ѣ�����������1��4-�ӳɵķ�ʽ���мӾۣ���Ӧ����ʽΪ
�����������Ϣ�ɷ��Ƶ�B��Ӧ����CH3CH=���ֺ�=CHC��CH3��=���֣�B��������H2�ӳɵõ�2-�����飬��B�Ľṹ��ʽΪCH3CH=CHC��CH3��=CHCH3��B�����Ӿ۷�Ӧʱ������̼̼˫�������ѣ�����������1��4-�ӳɵķ�ʽ���мӾۣ���Ӧ����ʽΪ ����B������HBr�ļӳɣ�B��HBrΪ���ԳƷ��ӣ��ɷ���1��2-�ӳɣ������֣�1��4-�ӳ������֣�3��4-�ӳ������֣�1��2��3��4-�ӳ���6�֣����Թ���12�֣�
����B������HBr�ļӳɣ�B��HBrΪ���ԳƷ��ӣ��ɷ���1��2-�ӳɣ������֣�1��4-�ӳ������֣�3��4-�ӳ������֣�1��2��3��4-�ӳ���6�֣����Թ���12�֣�
�ʴ�Ϊ��CH3CH=CHC��CH3��=CHCH3�� ��12��
��12��
��5������C������ʽC9H20������һ��ͬ���칹��C1��C2��C3ȴ�ֱ���ɶ���ֻ����1����Ӧ��ϩ����D1��D2��D3��������õ�����Aͬ���칹�������ڵ�����̼ԭ���Ϻ�����ԭ�ӵ�λ��ֻ��1�����������������A��ͬ���칹�壨C1��C2��C3��Ϊ��CH3��3C-C��CH3��2-CH2-CH3����CH3��2CH-C��CH3��2-CH��CH3��2��C��CH2-CH3��4�е�һ�֣�1molD3�������Ը��������Һ������ɲ���1molCO2��ͪ�����ʣ�����D3ΪCH3��3C-C��CH3��2-CH=CH2������C3ΪCH3��3C-C��CH3��2-CH2-CH3��������Ϊ2��3��3��4-�ļ����飬
�ʴ�Ϊ��2��3��3��4-�ļ����飮
���� �⿼���л���ļ������жϡ�ͬ���칹����ƶ�����д����ѧ����ʽ����д��֪ʶ������ʱע����������Ϣ���Ѷ��еȣ�������Ϣ�жϷ��������Ľṹ�ص��ǽ���ؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl | B�� | CS2 | C�� | H2S | D�� | SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �а�ɫ��״�����������Ҳ��ܽ� | B�� | ���а�ɫ��״�����������ܽ� | ||
| C�� | һֱ���������� | D�� | ���������а�ɫ��״���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� | 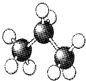 | C�� | 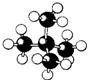 | D�� | 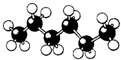 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ����Ļ�ѧʽ | CH3COOH | HCN | H2S |
| ���볣����25�棩 | 1.8��10-5 | 4.9��10-10 | K1=1.3��10-7 K2=7.1��10-15 |
| A�� | �����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��CH3COONa����pH��Na2S����pH��NaCN�� | |
| B�� | NaHS��Na2S�Ļ����Һ�У�һ������c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-�� | |
| C�� | a mol/LHCN��Һ��b mol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CN-������aһ��С�ڻ����b | |
| D�� | ijŨ�ȵ�NaCN��Һ��pH=d����������ˮ�������c��OH-��=10-dmol/L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com