

���� ���Ʊ����̿�֪��NaClO3��SO2��H2SO4�ữ����������ClO2������NaClO3�������������ղ���ΪNaHSO4��˵�������������ƣ��Ҳ���ClO2�����ݵ����غ��֪���˷�Ӧ�Ļ�ѧ����ʽΪ2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��ѡ��BaCl2��Һ��ȥS042-��NaOH��ȥʳ��ˮ�е�Mg2+��ѡ��̼���Ƴ�ȥʳ��ˮ�е�Ca2+��Ȼ����װ��������ClO2�õ�������ClO2-������Cl-ʧ��������Cl2�����������������������Һ����ClO2������ΪClO2-�����NaClO2��Һ�ᾧ������õ���Ʒ���Դ������
��� �⣺��1������Ӧ������������C1O2�Ļ�ѧ����ʽΪ��2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��
�ʴ�Ϊ��2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��
��2�����ڵ���ʳ��ˮ���ȳ�ȥ���е�Ca2+��Mg��S042-�����ʣ��������ù���BaCl2��Һ��ȥS042-�������ù���Na2CO3��Һ��ȥCa2+��Ba2+�������NaOH��Һ��ȥMg2+��CO32-��
�ʴ�Ϊ��BaCl2��
��3������������Ϊ50%˫��ˮ����30%��H202��Һ2kg����Ҫ�IJ�������������������ͷ�ιܡ��ձ�����Ͳ��
�ʴ�Ϊ����Ͳ��
��4��֤��NaClO2���������Եķ����ǣ���B����Һ���ȳ�ȥH2O2����������������ữ���ټ��뺬Fe2+�Ļ��������Fe3+���ɣ���֤��NaClO2���������ԣ���KSCN��Һ����Fe3+����������Һ��죻
�ʴ�Ϊ���ڣ��ݢޣ�
��5���ӳ�ƷҺ�еõ�NaC102•3H20����IJ�������Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
��6���ٷ�Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵������ζ��յ㣻
�ʴ�Ϊ���������һ��Na2S2O3��Һʱ����Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��
����NaC1O2��2I2��4S2O32-����25mL����Һ��n��NaC102��=$\frac{1}{4}$n��S2O32-��=$\frac{1}{4}$cV��10-3����Ʒ��Һ100mL������Ʒ��NaC1O2�����ʵ���ΪcV��10-3mol��
�ʴ�Ϊ��cV��10-3mol��
���� ������һ���������������еĹ�������Ϊ�����ʵ���⣬��Ҫ����ס�������̵Ĺ��̣��ҵ���������ͻ�ƿڣ����û���֪ʶ���������Ҫ�õ��IJ�Ʒ�������Ҫ���̲���ѧ���Ļ���֪ʶӦ�õ�ʵ�ʽ��������ȥ��Ҫ�����֪ʶ�����ι̣�ͬʱ���ܽ�����֪ʶӦ��ʵ��Ӧ���У�


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
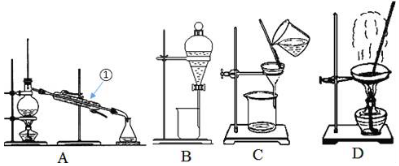
| A�� | ͼʾ���̱�ʾ�˵����������ƻ����� | |
| B�� | ͼ���漰���������Ϊ���������� | |
| C�� | �����������N���������� | |
| D�� | ͼʾ�������Ĺ������漰�ķ�Ӧ��Ϊ������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | K+��Cl-��NO3-��S2- | B�� | K+��Fe2+��I-��SO42- | ||
| C�� | Na+��Cl-��NO3-��SO42- | D�� | K+��Ba2+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ����Fe2+��Cu2+ | B�� | һ����Fe2+��Cu2+��������Fe3+ | ||
| C�� | һ����Fe2+��������Cu2+ | D�� | ֻ��Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba2+��Cl-��SO42-��K+ | B�� | Mg2+��SO42-��Na+��Cl- | ||
| C�� | H+��CO32-��Al3+��Cl- | D�� | K+��S O32-��NO3-��H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
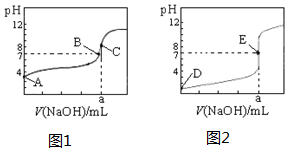 ��ͼΪ��������0.1000mol•L-1NaOH��Һ�ζ�20.00mL 0.1000mol•L-1�����20.00mL 0��.1000mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵������ȷ���ǣ�������
��ͼΪ��������0.1000mol•L-1NaOH��Һ�ζ�20.00mL 0.1000mol•L-1�����20.00mL 0��.1000mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵������ȷ���ǣ�������| A�� | ͼ2�ǵζ���������� | |
| B�� | ͼ1�ζ�ʱӦ��ѡ���̪��ָʾ�� | |
| C�� | B��ʱ����Ӧ������Һ�����V��NaOH����V��HA�� | |
| D�� | ��0 mL��V��NaOH����20.00 mLʱ����Ӧ��Һ�и�����Ũ�ȴ�С˳��һ����Ϊc��A-����c��Na+����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com