
ПВНјЦРAЎўBЎўCЎўDЎўEЎўFЎўGҫщОӘУР»ъ»ҜәПОпЎЈ

ўЕ DөД»ҜС§ГыіЖКЗ ЎЈ
ўЖ ·ҙУҰўЫөД»ҜС§·ҪіМКҪКЗЈә ЎЈ
ўЗ BөД·ЦЧУКҪКЗ Ј¬
AөДҪб№№јтКҪКЗЈәЎЎ ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ
ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ
·ҙУҰўЩөД·ҙУҰАаРНКЗ ЎЈ
ўИ·ыәППВБРИэёцМхјюөДBөДН¬·ЦТм№№МеөДКэДҝУР ёцЎЈ
ўЩә¬УРБЪ¶юИЎҙъұҪ»·Ҫб№№Ј»ўЪУлBҫЯУРПаН¬өД№ЩДЬНЕЈ»ўЫІ»ҝЙУлFeCl3ИЬТә·ўЙъПФЙ«·ҙУҰЎЈРҙіцЖдЦРЛщУРН¬·ЦТм№№МеөДҪб№№јтКҪЈЁИз№ыҙрМвЦҪЙПөДҝХёсІ»№»Ј¬ЗлЧФјәІ№МоНкХыЈ©Јә

ўЙGКЗЦШТӘөД№ӨТөФӯБПЈ¬УГ»ҜС§·ҪіМКҪұнКҫGөДТ»ЦЦЦШТӘ№ӨТөУГНҫ
ЎЈ

| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
| ЕЁБтЛб |
| Ўч |
| ЕЁБтЛб |
| Ўч |


 ИОТв1ЦЦ
ИОТв1ЦЦ ИОТв1ЦЦ
ИОТв1ЦЦІйҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә

| ЕЁH2SO4 |
| Ўч |
| ЕЁH2SO4 |
| Ўч |




| ҙЯ»ҜјБ |

| ҙЯ»ҜјБ |
| ҙЯ»ҜјБ |

| ҙЯ»ҜјБ |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә

| ЕЁH2SO4 |
| Ўч |
| ЕЁH2SO4 |
| Ўч |



| ҙЯ»ҜјБ |
 CH2-CH2
CH2-CH2 n»тCH2=CH2+H2O
n»тCH2=CH2+H2O| ҙЯ»ҜјБ |
| ҙЯ»ҜјБ |
 CH2-CH2
CH2-CH2 n»тCH2=CH2+H2O
n»тCH2=CH2+H2O| ҙЯ»ҜјБ |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2012-2013С§ДкЙҪ¶«КЎёЯИэ5ФВөЪТ»ҙОДЈДвҝјКФАнЧЫ»ҜС§КФҫнЈЁҪвОц°жЈ© МвРНЈәНЖ¶ПМв
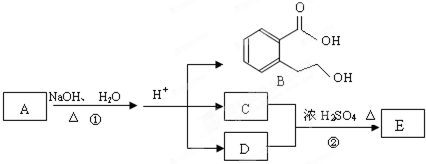
ПВНјЦРAЎўBЎўCЎўDЎўEҫщОӘУР»ъ»ҜәПОпЎЈТСЦӘЈәCДЬУлNaHCO3ИЬТә·ўЙъ·ҙУҰЈ¬C
әНDөДПа¶Ф·ЦЧУЦКБҝПаөИЈ¬ЗТDҙЯ»ҜСх»ҜөДІъОпІ»ДЬ·ўЙъТшҫө·ҙУҰЎЈ

»ШҙрПВБРОКМвЈә
ЈЁ1Ј©C·ЦЧУЦР№ЩДЬНЕөДГыіЖКЗ__________Ј»»ҜәПОпBІ»ДЬ·ўЙъөД·ҙУҰКЗ_________ЎЈ(МоЧЦДёРтәЕ) aЈ®јУіЙ·ҙУҰbЈ®Л®Ҫв·ҙУҰcЈ®ПыИҘ·ҙУҰdЈ®хҘ»Ҝ·ҙУҰ
ЈЁ2Ј©РҙіцEөДҪб№№јтКҪ______________________________________ЎЈ
ЈЁ3Ј©Рҙіц·ҙУҰўЩөД»ҜС§·ҪіМКҪЈә_____________________________________________ЎЈ
ЈЁ4Ј©Н¬Кұ·ыәППВБРИэёцМхјюөДBөДН¬·ЦТм№№МеУР¶аЦЦЈә
aЈ®ұҪ»·ЙПУР¶юёцИЎҙъ»щЗТұҪ»·ЙПөДТ»ВИҙъОпУР2ЦЦ
bЈ®ДЬУлFeCl3ИЬТә·ўЙъПФЙ«·ҙУҰ
cЈ®ДЬ·ўЙъЛ®Ҫв·ҙУҰ
КФРҙіцlmol BөДН¬·ЦТм№№МеЦРЈ¬ДЬУл3molNaOH·ҙУҰөДҪб№№јтКҪ_______________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013ҪмҪӯОчКЎёЯТ»ПВС§ЖЪЖЪЦРҝјКФ»ҜС§КФҫн МвРНЈәМоҝХМв
ЈЁ15·ЦЈ©ПВНјЦРAЎўBЎўCЎўDКЗН¬ЦЬЖЪ»тН¬ЦчЧеөДПаБЪФӘЛШЈә

ЈЁ1Ј©ТСЦӘЈәAФӘЛШөДЧоөНјЫОӘ-3јЫЈ¬ЛьөДЧоёЯјЫСх»ҜОпә¬Сх56.34ЈҘЈ¬ФӯЧУәЛДЪЦРЧУКэұИЦКЧУКэ¶а1ёцЈ¬ФтAФӘЛШФӯЧУөДЦКБҝКэОӘЎЎЎЎЎЎ ЎЎЈ¬ФӯЧУРтКэОӘ____ ___Ј¬AФӘЛШФЪФӘФӘЛШЦЬЖЪұнЦРөДО»ЦГОӘ___ __ЎЈ
ЈЁ2Ј©РҙіцФӘЛШ·ыәЕA__ ______B____ ____Ј¬CЎЎЎЎЎЎ ЎЎ ЎЎЈ¬D_____ ____ЎЈ
ЈЁ3Ј©AЎўBЎўCИэЦЦФӘЛШЧоёЯјЫСх»ҜОпөДЛ®»ҜОпөД»ҜС§КҪОӘЎЎЎЎЎЎЎЎЎЎЈ¬ЎЎЎЎЎЎЎЎЎЎЈ¬ЎЎЎЎЎЎЎЎЈ¬ЖдЦРЛбРФЧоЗҝөДКЗ_________ЎЈ
ЈЁ4Ј©BЎўDБҪЦЦФӘЛШәНЗвЧйіЙөДЖшМ¬Зв»ҜОпөД»ҜС§КҪТАҙООӘ__________Ј¬ЎЎЎЎЎЎЎЎЎЎЈ¬ЖдЦР______ __ОИ¶ЁРФЧоҙуЈ¬____ ____»№ФӯРФЧоЗҝЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com