
��֪����Ľṹ��ʽΪ�� 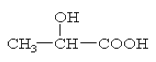
��1����90g���������������Ʒ�Ӧ�����������ڱ�״���µ������_____________L��
����ʽΪ ��
��֪�������������һ�������·�Ӧ���ɻ�״������д���÷�Ӧ�ķ���ʽ��
��
��2����֪�����ǿ��Ժʹ�����һ�������·���������Ӧ����1Ħ������������������
Ħ�����ᡣ
��3������ʽΪC6H12��ij���������е�̼ԭ�Ӷ�һ����ͬһƽ���ϣ�������Ľṹ��ʽΪ
__________________��
��4��ij����ͬϵ�����ʽΪC11H16��������ֻ��һ��ȡ��������ͬ���칹�干�� �֡�
��1��22.4 L CH2OHCH2COOH+2Na �� CH2(ONa)CH2COONa+H2��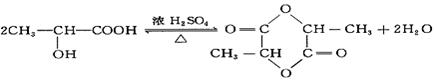
��2��5 ��3�� CH3-C(CH3)=C(CH3)-CH3 ��4�� 8
���������������1��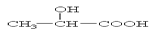 ����ȷ�������Ϊ180������90g����Ϊ0.5mol������Ӻ����Ȼ����ǻ��������Ƶķ�ӦΪ: CH2OHCH2COOH+2Na �� CH2(ONa)CH2COONa+H2��,����0.5mol�������������Ʒ�Ӧ����1mol���������ڱ�״���µ������22.4L��
����ȷ�������Ϊ180������90g����Ϊ0.5mol������Ӻ����Ȼ����ǻ��������Ƶķ�ӦΪ: CH2OHCH2COOH+2Na �� CH2(ONa)CH2COONa+H2��,����0.5mol�������������Ʒ�Ӧ����1mol���������ڱ�״���µ������22.4L�� ��һ�������·�Ӧ���ɻ�״�����Ļ�ѧ��Ӧ����ʽΪ��
��һ�������·�Ӧ���ɻ�״�����Ļ�ѧ��Ӧ����ʽΪ��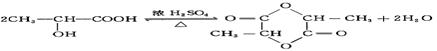 ����2�������ǿ��Ժʹ�����һ�������·���������Ӧ��һ�������Ƿ��Ӻ���5���ǻ�������1Ħ������������������5Ħ�������3������ʽΪC6H12��ij���������е�̼ԭ�Ӷ�һ����ͬһƽ���ϣ�������Ľṹ��ʽΪCH3-C(CH3)=C(CH3)-CH3����4��ij����ͬϵ�����ʽΪC11H16��������ֻ��һ��ȡ������������Щ������ֱ�Ϊ
����2�������ǿ��Ժʹ�����һ�������·���������Ӧ��һ�������Ƿ��Ӻ���5���ǻ�������1Ħ������������������5Ħ�������3������ʽΪC6H12��ij���������е�̼ԭ�Ӷ�һ����ͬһƽ���ϣ�������Ľṹ��ʽΪCH3-C(CH3)=C(CH3)-CH3����4��ij����ͬϵ�����ʽΪC11H16��������ֻ��һ��ȡ������������Щ������ֱ�Ϊ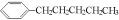 ��
�� ��
��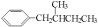 ��
��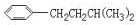 ��
��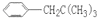 ��
�� ��
�� ��
��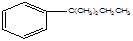 ��һ��8�֡�
��һ��8�֡�
���㣺���봼����ķ�Ӧ��ͬ���칹��
���������⿼�������봼����ķ�Ӧ��ͬ���칹�壬�����ۺ��Խϸߣ��ر������һ��С�⣬�����˱���ͬϵ���ͬ���칹�����д����С���ѶȺܴ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
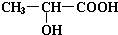 ���Իش�
���Իش�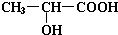 +2Na��
+2Na��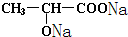 +H2��
+H2��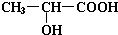 +2Na��
+2Na��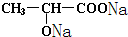 +H2��
+H2��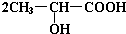 +Na2CO3��
+Na2CO3�� +H2O+CO2��
+H2O+CO2��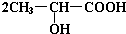 +Na2CO3��
+Na2CO3�� +H2O+CO2��
+H2O+CO2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
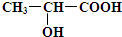 ���Իش�
���Իش� -
- -COOH
-COOH -
- -COOH
-COOH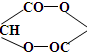 CH-CH3
CH-CH3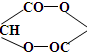 CH-CH3
CH-CH3�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

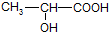
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
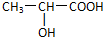
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣���֪����Ľṹ��ʽΪ ���Իش�
��1����������к���________��_________���ֹ����ţ�д���ƣ���
��2�����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ
___________________________________________________________________��
��3��������Na2CO3��Һ��Ӧ�Ļ�ѧ����ʽΪ
___________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com