

·ÖĪö £Ø1£©ŃĒĢśĄė×Ó¾ßÓŠ»¹ŌŠŌ£¬Äܱ»øßĆĢĖį¼ŲŃõ»ÆĪŖČż¼ŪĢś£¬Ź¹øßĆĢĖį¼ŲĶŹÉ«£»
£Ø2£©øł¾ŻH2O2+2Fe2++2H+=2Fe3++2H20µÄ²śĪļ½ā“š£»
£Ø3£©“ÓČÜŅŗÖŠŅŖĪö³ö¾§Ģ壬²ÉÓĆĄäČ“½į¾§·Ø£»¹żĀĖŹ±ŅŖÓƵ½²£Į§°ōŅżĮ÷£»
½ā“š ½ā£ŗ£Ø1£©ŃĒĢśĄė×Ó¾ßÓŠ»¹ŌŠŌ£¬Äܱ»øßĆĢĖį¼ŲŃõ»ÆĪŖČż¼ŪĢśĄė×Ó£¬Ź¹øßĆĢĖį¼ŲĶŹÉ«£¬ŹĒ¼ģŃéČÜŅŗAÖŠFe2+µÄ×ī¼ŃŹŌ¼Į£¬
¹Ź“š°øĪŖ£ŗa£»
£Ø2£©H2O2¾ßÓŠŃõ»ÆŠŌ£¬ŌŚøĆ·“Ó¦ÖŠ×÷Ńõ»Æ¼Į£¬ŃĒĢśĄė×Ó¾ßÓŠ»¹ŌŠŌ£¬·¢ÉśH2O2+2Fe2++2H+=2Fe3++2H20£¬H2O2ÓėFe2+·“Ӧɜ³ÉµÄH20ĪŖČܼĮĪŽĪŪČ¾£¬²»»įŅżČėŠĀµÄŌÓÖŹĄė×Ó£¬
¹Ź“š°øĪŖ£ŗH2O2+2Fe2++2H+=2Fe3++2H20£»²»»įŅżČėŠĀµÄŌÓÖŹĄė×Ó£»
£Ø3£©ÓÉČÜŅŗÖĘČ”¾§Ģ壬Šč¾¹ż¼ÓČČÅØĖõ£¬ĄäČ“½į¾§²ÅæɵƵ½£¬ŌŚ¹żĀĖ²Ł×÷ÖŠ£¬³żÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā£¬»¹ŠčµÄŅ»ÖÖ²£Į§ŅĒĘ÷ŹĒ²£Į§°ō£¬ĖüµÄ×÷ÓĆŹĒŅżĮ÷£¬
¹Ź“š°øĪŖ£ŗĄäČ“½į¾§£»ŅżĮ÷£®
µćĘĄ ±¾Ģā漲鳣¼ū½šŹōµÄµ„ÖŹ¼°Ęä»ÆŗĻĪļµÄÓ¦ÓĆŗĶĮņĖįĶ½į¾§Ė®ŗ¬ĮæµÄ²ā¶Ø£¬ÕĘĪÕĪļÖŹµÄÖĘČ”ŗĶĢį“攢Ąė×ӵļģŃéµČŹµŃé²Ł×÷£¬·ÖĪöĶ¼ĻóŠÅĻ¢ŹĒĶź³É±¾ĢāÄæµÄ¹Ų¼ü£¬ĢāÄæ½ĻĪŖ×ŪŗĻ£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆĀĮ | B£® | ½šøÕŹÆ | C£® | ¶žŃõ»ÆĢ¼ | D£® | ¶žŃõ»Æ¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 4£ŗ1 | B£® | 1£ŗ4 | C£® | 1£ŗ6 | D£® | 6£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹÆÄ« | B£® | ¶žŃõ»Æ¹č | C£® | Ńõ»ÆĆ¾ | D£® | ¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| Ń”Ļī | ÄæµÄ | ²Ł×÷ |
| A | ÅäÖĆ100mL 1.0mol•L-1CuSO4ČÜŅŗ | ½«25gCuSO4•5H2OČÜÓŚ100mLÕōĮóĖ®ÖŠ |
| B | ³żČ„KNO3¹ĢĢåÖŠÉŁĮæNaCl | ½«»ģŗĻĪļÖĘ³ÉŹģµÄ±„ŗĶČÜŅŗ£¬ĄäČ“½į¾§£¬¹żĀĖ |
| C | ĢįČ”äåĖ®ÖŠµÄBr2 | ĻņČÜŅŗÖŠ¼ÓČėŅŅ“¼ŗóÕńµ“£¬¾²ÖĆ£¬·ÖŅŗ |
| D | ¼ģŃéČÜŅŗÖŠŹĒ·ńŗ¬ÓŠNH4+ | ȔɣĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČėNaOHŗ󣬼ÓČČ£¬ŌŚŹŌ¹ÜæŚ·ÅÖĆ Ņ»æéŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Cl2 | B£® | CaCl2 | C£® | Ca£ØOH£©2 | D£® | Ca£ØClO£©2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | äåµ„ÖŹ£ŗBr | B£® | ĀČĄė×ӵĽį¹¹Ź¾ŅāĶ¼£ŗ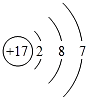 | ||
| C£® | H2OµÄ½į¹¹Ź½£ŗ | D£® | CaCl2µÄµē×ÓŹ½£ŗ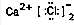 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
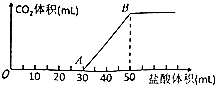 ½«5.04gNa2CO3”¢NaOHµÄ¹ĢĢå»ģŗĻĪļ¼ÓĖ®Čܽā£¬ĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė2mol•L-1µÄŃĪĖį£¬Ėł¼ÓŃĪĖįµÄĢå»żÓė²śÉśCO2µÄĢå»ż£Ø±ź×¼×“æö£©¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©
½«5.04gNa2CO3”¢NaOHµÄ¹ĢĢå»ģŗĻĪļ¼ÓĖ®Čܽā£¬ĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė2mol•L-1µÄŃĪĖį£¬Ėł¼ÓŃĪĖįµÄĢå»żÓė²śÉśCO2µÄĢå»ż£Ø±ź×¼×“æö£©¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | OA¶Ī·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗH++OH-ØTH2O CO32-+H+ØTHCO3- | |
| B£® | BµćČÜŅŗÖŠµÄČÜÖŹĪŖNaCl£¬ĘäÖŹĮæĪŖ5.85g | |
| C£® | µ±¼ÓČė35mLŃĪĖįŹ±£¬²śÉśCO2µÄĢå»żĪŖ224mL£Ø±ź×¼×“æö£© | |
| D£® | »ģŗĻĪļÖŠNaOHµÄÖŹĮæ2.40g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com