
N2(g)+2O2(g)![]() 2NO2(g) ��H1=+67.7 kJ�� mol-1
2NO2(g) ��H1=+67.7 kJ�� mol-1
N2H4(g)+O2(g)![]() N2(g)+2H2O(g) ��H2=-534 kJ�� mol-1
N2(g)+2H2O(g) ��H2=-534 kJ�� mol-1
�Լ���1 mol�ºͶ���������ȫ��Ӧʱ�ų�������Ϊ______________kJ��д���������������Ӧ���Ȼ�ѧ����ʽ__________________________________________��
���������ݸ�˹���ɣ��ڷ�Ӧ������ȥO2�ɵõ�N2H4��NO2�ķ�Ӧ,��ڡ�2-�ٵ� 2N2H4(g)-N2(g)![]() 2N2(g)+4H2O(g)-2NO2(g)
2N2(g)+4H2O(g)-2NO2(g)
�����ã�2N2H4(g)+2NO2(g)![]() 3N2(g)+4H2O(g) ��H=2��H2-��H1=-534 kJ mol-1��2-67.7 kJ�� mol-1=-1 135.7 kJ mol-1��
3N2(g)+4H2O(g) ��H=2��H2-��H1=-534 kJ mol-1��2-67.7 kJ�� mol-1=-1 135.7 kJ mol-1��
��1 mol N2H4��Ӧ�ų�������Ϊ![]() =567.85 kJ
=567.85 kJ
�𰸣�567.85 2N2H4(g)+2NO2(g)![]() 3N2(g)+4H2O(g) ��H=-1 135.7 kJ��mol-1
3N2(g)+4H2O(g) ��H=-1 135.7 kJ��mol-1



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
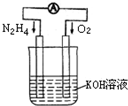 ���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮
���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮| 10-9 |
| a-0.01 |
| 10-9 |
| a-0.01 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com