
����Ŀ�����仯������������;����ش��������⡣
(1)��̬�����Ӽ۲���ӵĹ������ʽΪ___________���������������ܼ��Ĺ����Ϊ___________��
(2)��������������е�����(S8)��SO2��Na2S��K2S�ȣ��������ʵ��۵��ɸߵ��͵�˳������Ϊ___________��ԭ����___________��
(3)��Ǧ��(����Ǧ)��һ�ֱȽϳ����Ŀ�����ܷ�ӦΪ��PbS+4HCl(Ũ)=H2[PbCl4]+H2S������H2[PbCl4]����λԭ����_________����һ������I1(Cl)___________I1(S)(����>������<������=��)��H2S������ӻ���ʽΪ_______�����з��ӿռ�Ĺ�����H2S��ͬ����___________��
A��H2O B��SO3 C��O3 D��CH4
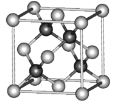
(4)��Ǧ�������������ͼ��ʾ�������Ӳ�ȡ���������ѻ���Ǧ�����������������γɵ�___________��϶�С���֪�����ܶ�Ϊ��g��cm��3�������ӵ�������ֵΪNA��������������Ǧ��������ľ���Ϊ___________nm��
���𰸡�![]() 3 Na2S��K2S��S8��SO2 Na2S��K2S��Ϊ���Ӿ��壬�۵�ϸߣ�Na+�뾶��K+�뾶С����Na2S�۵�ߣ�S8��SO2��Ϊ���Ӿ��壬�۵�ϵͣ���S8����Է���������SO2��S8�۵��SO2�� Cl �� sp3 AC ������
3 Na2S��K2S��S8��SO2 Na2S��K2S��Ϊ���Ӿ��壬�۵�ϸߣ�Na+�뾶��K+�뾶С����Na2S�۵�ߣ�S8��SO2��Ϊ���Ӿ��壬�۵�ϵͣ���S8����Է���������SO2��S8�۵��SO2�� Cl �� sp3 AC ������ ![]() ��
��![]() ��107
��107
��������
(1)��Ϊ16��Ԫ�أ������Ӻ�����18�����ӣ��ݴ���д��̬�����Ӽ۲���ӵĹ������ʽ�����ж�����ܼ���ԭ�ӹ������
(2) Na2S��K2S��Ϊ���Ӿ��壬S8��SO2��Ϊ���Ӿ��壬�����۷е���жϷ����������
(3) H2[PbCl4]����λԭ����Cl������ԭ����Pb��ͬһ���ڣ������ң���һ�����ܳ�����������ƣ����ݼ۲���Ӷ����ļ��㹫ʽ�����жϣ�
(4)���ݷ�Ǧ�����������ͼ�������һ�������к��е�Ǧ���Ӻ������ӵ���Ŀ��������������������Ӷ����㾧�����ⳤ����������������Ǧ��������ľ���Ϊ������Խ��ߵ�![]() ���ݴ˽��
���ݴ˽��
(1)��Ϊ16��Ԫ�أ������Ӻ�����18�����ӣ���̬�����Ӽ۲���ӵĹ������ʽΪ![]() ���������������ܼ�Ϊ3p������3�����ֱ��ԭ�ӹ�����ʴ�Ϊ��
���������������ܼ�Ϊ3p������3�����ֱ��ԭ�ӹ�����ʴ�Ϊ��![]() ��3��
��3��
(2) Na2S��K2S��Ϊ���Ӿ��壬�۵�ϸߣ�Na+�뾶��K+�뾶С����Na2S�۵�ߣ�S8��SO2��Ϊ���Ӿ��壬�۵�ϵͣ���S8����Է���������SO2��S8�۵��SO2�ߣ�����������ʵ��۵��ɸߵ��͵�˳������ΪNa2S��K2S��S8��SO2���ʴ�Ϊ��Na2S��K2S��S8��SO2��Na2S��K2S��Ϊ���Ӿ��壬�۵�ϸߣ�Na+�뾶��K+�뾶С����Na2S�۵�ߣ�S8��SO2��Ϊ���Ӿ��壬�۵�ϵͣ���S8����Է���������SO2��S8�۵��SO2�ߣ�
(3) H2[PbCl4]����λԭ����Cl������ԭ����Pb��ͬһ���ڣ������ң���һ�����ܳ�����������ƣ�I1(Cl)��I1(S)��H2S����ļ۲���Ӷ���=2+![]() =4������sp3�ӻ���ΪV�η��ӣ�A��H2O��O�ļ۲���Ӷ���=2+
=4������sp3�ӻ���ΪV�η��ӣ�A��H2O��O�ļ۲���Ӷ���=2+![]() =4������sp3�ӻ���ΪV�η��ӣ�B��SO3����ļ۲���Ӷ���=3+
=4������sp3�ӻ���ΪV�η��ӣ�B��SO3����ļ۲���Ӷ���=3+![]() =3������sp2�ӻ���Ϊƽ�������η��ӣ�C��O3��O�ļ۲���Ӷ���=2+
=3������sp2�ӻ���Ϊƽ�������η��ӣ�C��O3��O�ļ۲���Ӷ���=2+![]() =3������sp2�ӻ���ΪV�η��ӣ�D��CH4��C�ļ۲���Ӷ���=4+
=3������sp2�ӻ���ΪV�η��ӣ�D��CH4��C�ļ۲���Ӷ���=4+![]() =4������sp3�ӻ���Ϊ���������η��ӣ��ռ�Ĺ�����H2S�����AC���ʴ�Ϊ��Cl������sp3��AC��
=4������sp3�ӻ���Ϊ���������η��ӣ��ռ�Ĺ�����H2S�����AC���ʴ�Ϊ��Cl������sp3��AC��
(4)���ݷ�Ǧ�����������ͼ�������Ӳ�ȡ���������ѻ���Ǧ�����������������γɵ��������϶�С�һ�������к���4��Ǧ���ӣ������ӵ���Ŀ=8��![]() +6��
+6��![]() =4������������=
=4������������=![]() g=
g=![]() g�������ⳤ=
g�������ⳤ=![]() cm����������������Ǧ��������ľ���Ϊ������Խ��ߵ�
cm����������������Ǧ��������ľ���Ϊ������Խ��ߵ�![]() =
=![]() ��
��![]() cm=
cm=![]() ��
��![]() ��107nm���ʴ�Ϊ�������壻
��107nm���ʴ�Ϊ�������壻![]() ��
��![]() ��107��
��107��


 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ����ᶡ����װ����ͼ��ʾ�����з����������

A. ����������������߶�����ת����
B. �ᴿ���ᶡ����Ҫ����ˮ������������Һϴ��
C. ������a����������������
D. �����ᶡ���ķ�Ӧ�¶ȳ���100�治����ˮԡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ҫ�Ĺ���Ԫ�ء�
(1)��λ��Ԫ�����ڱ��������ڢ��壬���̬ԭ����δ�ɶԵ��Ӹ���Ϊ___________��
(2)��̬Fe3+�ĺ�������Ų�ʽ___________
(3)��������һ�ִ��Բ��ϣ���ҵ���Ʊ�ʱ������ˮ�ⷨ���Ʊ�ʱ����������(CO(NH2)2)�������Ƶȼ������ʡ����ط����������ǽ���Ԫ�صĵ縺���ɴ�С��˳����___________����������������������Ŀ֮��Ϊ___________����������̼ԭ�ӵ��ӻ�����___________��
(4)������Ҳ��ʹ�ó��������Ʊ�ʱ�����백(NH3)������(N2H4)�������֪��(NH3�۵㣺��77.8%�桢�е㣺��33.5%��)������(N2H4�۵㣺2�桢�е㣺113.5��C)�������۷е�ߵ͵���Ҫԭ��______________________��
(5)Co(NH3)5BrSO4���γ������ܵ�������֪Co3+����λ��Ϊ6��Ϊȷ����������Ľṹ���ֶ�����������������ʵ�飺�ڵ�һ���������Һ�м�����������Һ������ɫ���������һ������������Ϊ___________���ڵڶ����������Һ�м�����������Һ��������ɫ��������ڶ�������������Ϊ___________��

(6)��������̼�ܽ���r-Fe���γɵ�һ�ּ�϶�����壬���ԣ��侧��Ϊ���������ṹ������ͼ��ʾ��������ʵĻ�ѧʽΪ___________����Ʒ���ܶ�Ϊdg��cm��3���������������̼ԭ�ӵľ���Ϊ___________pm(�����ӵ�������ֵ��NA��ʾ��д����ļ���ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����н�����ʹ�����������õ�ҩ��ұصá���Ҫ�ɷֵĽṹ��ʽ��ͼ���������� ��
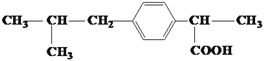
�ٷ����廯���� ��֬���廯���� ���л����� ���л��߷��ӻ����� �ݷ�����
A. �ۢ� B. �ڢ� C. �٢� D. �٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(NaNO2)��һ������Ʒ�����г�����ʳƷ���Ӽ���ʹ��ʱ�����ϸ������������ij��ȤС�����������ͼ��ʾ��װ���Ʊ�NaNO2(A�м���װ������ȥ��NO����������Ʒ�ĩ�������Ϸ�Ӧ��Ҳ�ܱ�����KMnO4������NO3��)��

(1)����a��������___________��
(2)A��ʵ������Ϊ___________��
(3)Ϊ��֤�Ƶõ��������ƵĴ��ȣ�Cװ����ʢ�ŵ��Լ�������___________(����ĸ���)��
A��P2O5 B����ˮCaCl2 C����ʯ�� D��Ũ����
(4)E�з�����Ӧ�����ӷ���ʽΪ___________��
(5)����ߵ�ԭ�������ʵĽǶȳ���������Bװ����ƴ���һ��ȱ�ݣ���θĽ�?_______��
(6)��֪��2NO2��+2I��+4H+=2NO��+I2+2H2O��2S2O32��+I2=2I��+S4O62��
Ϊ�ⶨ�õ���Ʒ��NaNO2�Ĵ��ȣ���ȡ����ʵ�鲽�裺ȷ��ȡ����Ϊ1.00g��NaNO2��Ʒ������ƿ�У�������ˮ�ܽ���������0.800mol��L��1KI��Һ��������Һ��Ȼ��μ�ϡ�����ַ�Ӧ����0.500mol��L��1Na2S2O3��Һ�ζ����յ㣬�������ظ����ϲ�����3��������Na2S2O3��Һ������ֱ�Ϊ20.02mL��19.98mL��20.25 mL���ζ��յ�ʱ��ʵ������____������Ʒ��NaNO2����Ϊ_____(����һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���Ȼ�菉���ľ���(�����е���С�ظ���Ԫ)����֪���������������Cs�����Ӻ˼����Ϊa cm���Ȼ�蘆���Է�������ΪM��NAΪ�����ӵ����������Ȼ�菉����ܶ���(����)
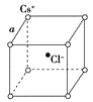
A. ![]() g/cm3 B.
g/cm3 B. ![]() g/cm3 C.
g/cm3 C. ![]() g/cm3 D.
g/cm3 D. ![]() g/cm3
g/cm3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)��һ�ָ�Ч��ˮ��.��֪:K2FeO4 ������ˮ,����Һ����ɫ������ŨKOH ��Һ,��0����5����ǿ������Һ�н��ȶ�.ijС��ͬѧ����ͼװ���Ʊ���̽��
K2FeO4 ������.�Ʊ�ԭ��:
3Cl2��2Fe(OH)3��10KOH��2K2FeO4��6KCl��8H2O,װ����ͼ��ʾ(�г�װ����)
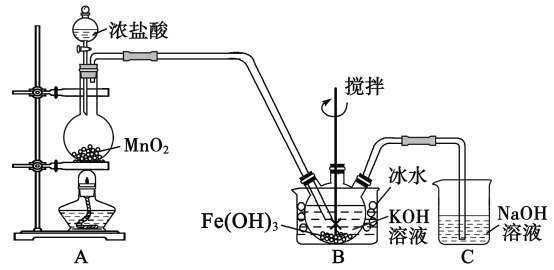
(1)ʢ�Ŷ������̵���������___________________,װ��C��������____________________��
(2)װ��A �з�Ӧ�Ļ�ѧ����ʽ��________________________________________��
(3)ʵ��ʱ���ñ�ˮԡ��ԭ����____________________,��װ�ô���һ������ȱ��,��ָ��____________��
(4)K2FeO4 �ֲ�Ʒ����Fe(OH)3��KCl������,һ����75���Ҵ�����ϴ��,��Ŀ����_____________________��
(5)��������֪,K2FeO4 �ܽ� Mn2�������� MnO4��.��С���������ʵ�������֤:
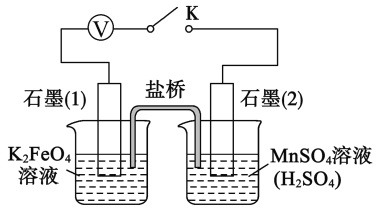
�ر�K,���ձ���Һ���ɫ,���ձ���Һ����ɫ.��������ձ���Һ�ʻ�ɫ��ԭ��,��Ҫ���Լ���__________��д��K2FeO4 ����Mn2�� �����ӷ���ʽ: ___________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����в�����������������ǣ� ��
A. ��Ϊ��ȡʱ��������������Ӧ��������
B. ��Ϊ��ʽ�ζ��������������������¼��ʼ����
C. ��Ϊ��������ζ�IJ����������ж��������ܽ��ǿ״ս�ƿ��
D. ��Ϊ������Һ������ҡ�ȵIJ�����ҡ�Ⱥ��������Һ����ڿ̶���Ҳ�����ټ�ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ��mol��1���ش��й��кͷ�Ӧ�����⡣
(1)��0.1 mol Ba(OH)2���ϡ��Һ������ϡ���ᷴӦ���ܷų�________________kJ������
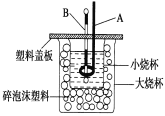
(2)��ͼװ��������A��������____________��������__________________________��
����ĭ���ϵ�������_____________________________��
Ҫ�ظ���������ʵ���Ŀ���� ____________________��
(3)��ͨ��ʵ��ⶨ�к��ȵĦ�H�������������ڣ�57.3 kJ��mol��1����ԭ������� ____________��
(4)����ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��__________(�ƫ����ƫС��������Ӱ�족)��
(5)��V1 mL 1.00 mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)������������ȷ������____��

A������ʵ��ʱ�����¶�Ϊ22 ��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C��NaOH��Һ��Ũ��ԼΪ1.00 mol��L��1
D����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com