
£®£Ø8·Ö£©²ŻĖįŃĒĢś¾§Ģå£ØFeC2O4”¤2H2O£©³£ÓĆ×÷·ÖĪöŹŌ¼Į¼°ĻŌÓ°¼ĮµČ”£ĻĀĶ¼ŹĒ½«Ņ»¶ØÖŹĮæµÄ²ŻĖįŃĒĢśŌŚė²Ęų·ÕĪ§ÖŠ½ųŠŠČČÖŲ·ÖĪöµÄŹ¾ŅāĶ¼£ØTG%±ķŹ¾²ŠĮō¹ĢĢåÖŹĮæÕ¼Ōѳʷ֏ĮæµÄ°Ł·ÖŹż£©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©B“¦²ŠĮōĪļµÄ»ÆѧŹ½ĪŖ ”£C“¦²ŠĮōĪļµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©A”śCÕūøö·“Ó¦¹ż³ĢÖŠ×Ü·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ÉĻŹöFeC2O4”¤2H2OŌŚė²ĘųĘų·ÕÖŠ½ųŠŠČČÖŲ·ÖĪöµÄŌŅņŹĒ ”£
Čō½«·Ö½āµĆµ½µÄ600”ꏱµÄ¹ĢĢåÓė×ćĮæµÄÅØĮņĖį·“Ó¦ŗ󣬽«ČÜŅŗÅØĖõ”¢ĄäČ“£¬ÓŠ“ų9øö½į¾§Ė®µÄ¾§ĢåĪö³ö£¬øĆ¾§ĢåµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©ĻÖČ”1.44gFeC2O4·ÅŌŚÄ³ÕęæÕµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŁ³äČė0.04molCO£¬¼ÓČČÖĮ1100”ę£¬ĘäÖŠ·“Ó¦£ŗFeO(s) + CO(g)  Fe(s) + CO2(g)µÄĘ½ŗā³£ŹżK=1/3£¬Ōņ·“Ó¦“ļĘ½ŗāŹ±FeOµÄ×Ŗ»ÆĀŹĪŖ ”£
Fe(s) + CO2(g)µÄĘ½ŗā³£ŹżK=1/3£¬Ōņ·“Ó¦“ļĘ½ŗāŹ±FeOµÄ×Ŗ»ÆĀŹĪŖ ”£
 ĶõŗóŠŪѧ°ø½Ģ²ÄĶźČ«½ā¶ĮĻµĮŠ“š°ø
ĶõŗóŠŪѧ°ø½Ģ²ÄĶźČ«½ā¶ĮĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

 ÕāŃł±ćµĆµ½ĮĖøß“æ¶ČµÄÄÉĆ×Ģś·Ū£®
ÕāŃł±ćµĆµ½ĮĖøß“æ¶ČµÄÄÉĆ×Ģś·Ū£®
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā


c[Cu(NH3
| ||
| c(Cu2+)?c4(NH3) |
| 1 |
| 3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ°²»ÕŹ””°½ÄĻŹ®Š£”±øßČż3ŌĀĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
²ŻĖįŃĒĢś¾§Ģå(FeC2O4”¤2H2O)ÓĆ×÷·ÖĪöŹŌ¼Į¼°ĻŌÓ°¼ĮŗĶŠĀŠĶµē³Ų²ÄĮĻĮ×ĖįŃĒĢśļ®µÄÉś²ś”£»Ų“šĻĀĮŠĪŹĢā£ŗ
I£®ŠĖȤŠ”×é¶Ō²ŻĖįŃĒĢś¾§ĢåµÄ·Ö½ā²śĪļ½ųŠŠŹµŃéŗĶĢ½¾æ”£Ģ½¾æ·Ö½āµĆµ½µÄ¹ĢĢå²śĪļÖŠĢśŌŖĖŲµÄ“ęŌŚŠĪŹ½”£
£Ø1£©Ģį³ö¼ŁÉč
¼ŁÉčŅ»£ŗ___________£»??? ¼ŁÉ趞£ŗČ«²æŹĒFeO £»?????? ¼ŁÉčČż£ŗFeOŗĶFe»ģŗĻĪļ”£
£Ø2£©Éč¼ĘŹµŃé·½°øÖ¤Ć÷¼ŁÉčČż”£
ŹµŃé²½Öč | ĻÖĻóÓė½įĀŪ |
²½Öč1£ŗĻņŹŌ¹ÜÖŠ¼ÓČėÉŁĮæ¹ĢĢå²śĪļ£¬ŌŁ¼ÓČė×ćĮæ????????? £¬³ä·ÖÕšµ“ | ČōČÜŅŗŃÕÉ«Ć÷ĻŌøıą£¬ĒŅÓŠ??????? Éś³É£¬ŌņÖ¤Ć÷ÓŠĢśµ„ÖŹ“ęŌŚ |
²½Öč2£ŗ½«²½Öč1ÖŠµĆµ½µÄ×ĒŅŗ¹żĀĖ£¬²¢ÓĆÕōĮóĖ®Ļ“µÓÖĮĻ“µÓŅŗĪŽÉ« |
|
²½Öč3£ŗČ„²½Öč2µĆµ½ÉŁĮæ¹ĢĢåÓėŹŌ¹ÜÖŠ£¬µĪ¼Ó
|
|
ĻŽŃ”ŹŌ¼Į£ŗĻ”ŃĪĖį”¢ŠĀÖʵÄĀČĖ®”¢0.1mol£®L-1CuSO4ČÜŅŗ”¢20% KSCNČÜŅŗ”¢ÕōĮóĖ®”£
¢ņ£®ŠĖȤŠ”×éŌŚĪÄĻ×ÖŠ²éŌĵ½£¬FeC2O4”¤2H2OŹÜČČ·Ö½āŹ±£¬¹ĢĢåÖŹĮæĖęĪĀ¶Č±ä»ÆµÄĒśĻßČēĻĀĶ¼ĖłŹ¾£¬Š“³ö¼ÓČȵ½400”ꏱ£¬FeC2O4”¤2H2O¾§ĢåŹÜČČ·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ_______________
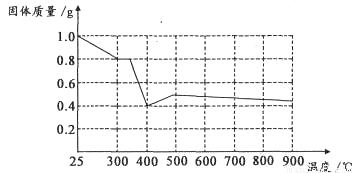
øł¾ŻĶ¼Ļó£¬ČēÓŠ1.0g²ŻĖįŃĒĢś¾§ĢåŌŚŪįŪöÖŠ³ØæŚ³ä·Ö¼ÓČČ£¬×īÖÕ²ŠĮōŗŚÉ«¹ĢĢåµÄÖŹĮæ“óÓŚ0.4g”£Ä³Ķ¬Ń§ÓÉ“ĖµĆ³ö½įĀŪ£ŗ¼ŁÉ趞²»³ÉĮ¢”£ÄćŹĒ·ńĶ¬ŅāøĆĶ¬Ń§µÄ½įĀŪ£¬²¢¼ņŹöĄķÓÉ£ŗ______________________”£????????????
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗŚĮś½Ź”øßČżÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£®£Ø8·Ö£©²ŻĖįŃĒĢś¾§Ģå£ØFeC2O4”¤2H2O£©³£ÓĆ×÷·ÖĪöŹŌ¼Į¼°ĻŌÓ°¼ĮµČ”£ĻĀĶ¼ŹĒ½«Ņ»¶ØÖŹĮæµÄ²ŻĖįŃĒĢśŌŚė²Ęų·ÕĪ§ÖŠ½ųŠŠČČÖŲ·ÖĪöµÄŹ¾ŅāĶ¼£ØTG%±ķŹ¾²ŠĮō¹ĢĢåÖŹĮæÕ¼Ōѳʷ֏ĮæµÄ°Ł·ÖŹż£©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©B“¦²ŠĮōĪļµÄ»ÆѧŹ½ĪŖ ”£C“¦²ŠĮōĪļµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©A”śCÕūøö·“Ó¦¹ż³ĢÖŠ×Ü·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ÉĻŹöFeC2O4”¤2H2OŌŚė²ĘųĘų·ÕÖŠ½ųŠŠČČÖŲ·ÖĪöµÄŌŅņŹĒ ”£
Čō½«·Ö½āµĆµ½µÄ600”ꏱµÄ¹ĢĢåÓė×ćĮæµÄÅØĮņĖį·“Ó¦ŗ󣬽«ČÜŅŗÅØĖõ”¢ĄäČ“£¬ÓŠ“ų9øö½į¾§Ė®µÄ¾§ĢåĪö³ö£¬øĆ¾§ĢåµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©ĻÖČ”1.44gFeC2O4·ÅŌŚÄ³ÕęæÕµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŁ³äČė0.04molCO£¬¼ÓČČÖĮ1100”ę£¬ĘäÖŠ·“Ó¦£ŗFeO(s) + CO(g)  Fe(s) + CO2(g)µÄĘ½ŗā³£ŹżK=1/3£¬Ōņ·“Ó¦“ļĘ½ŗāŹ±FeOµÄ×Ŗ»ÆĀŹĪŖ ”£
Fe(s) + CO2(g)µÄĘ½ŗā³£ŹżK=1/3£¬Ōņ·“Ó¦“ļĘ½ŗāŹ±FeOµÄ×Ŗ»ÆĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com