
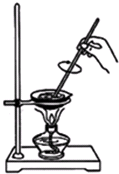
 ÖĘČ”·ŹŌķµÄŹµŃéÓŠŅŌĻĀ²½Öč£ŗ
ÖĘČ”·ŹŌķµÄŹµŃéÓŠŅŌĻĀ²½Öč£ŗ +3NaOH
+3NaOH| Ė® |
| ”÷ |
 £¬
£¬ +3NaOH”ś3C17H35COONa+
+3NaOH”ś3C17H35COONa+


 ±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ±±¾©ŹŠ³ÆŃōĒų2006”«2007ѧğµŚŅ»Ń§ĘŚĘŚÄ©Ķ³Ņ»æ¼ŹŌ”¢øßČż»ÆѧŹŌ¾ķ ĢāŠĶ£ŗ058
| |||||||||||||||||||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ŁŌŚŅ»øöøÉŌļµÄÕō·¢ĆóÖŠ¼ÓČėÖ²ĪļÓĶ8 mL”¢ŅŅ“¼8 mL”¢NaOHČÜŅŗ4 mL
¢ŚŌŚ²»¶Ļ½Į°čĻĀ£¬øųÕō·¢ĆóÖŠŅŗĢåĪ¢Ī¢¼ÓČČ£¬Ö±µ½»ģŗĻĪļ±ä³ķ
¢Ū¼ĢŠų¼ÓČČ£¬Ö±µ½°ŃŅ»µĪ»ģŗĻĪļ¼Óµ½Ė®ÖŠŹ±ŌŚŅŗĢå±ķĆę²»ŌŁŠĪ³ÉÓĶµĪĪŖÖ¹
¢Ü½«Ź¢»ģŗĻĪļµÄÕō·¢Ćó·ÅŌŚĄäĖ®ÖŠĄäČ“”£ÉŌ“żĘ¬æĢ£¬Ļņ»ģŗĻĪļÖŠ¼Ó20 mL ČČÕōĮóĖ®£¬ŌŁ·ÅŌŚĄäĖ®Ō”ÖŠĄäČ“”£Č»ŗó¼ÓČė25 mL NaCl±„ŗĶČÜŅŗ³ä·Ö½Į°č
¢ŻÓĆÉ“²¼ĀĖ³ö¹ĢĢåĪļÖŹ£¬ĘśČ„ĀĖŅŗ”£°Ń¹ĢĢåĪļÖŹ¼·øÉ”¢Ń¹³ÉĢõד”¢ĮĄøÉ£¬¼“µĆ·ŹŌķ”£
øł¾ŻŹµŃ飬ĢīæÕ£ŗ
£Ø1£©ČōŅŌ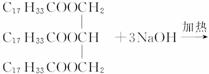 “ś±ķÖ²ĪļÓĶ£¬øĆĪļÖŹµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ__________£»Š“³öÓĆøĆĪļÖŹÖĘ·ŹŌķµÄ»Æѧ·“Ó¦·½³ĢŹ½_________________________________________”£
“ś±ķÖ²ĪļÓĶ£¬øĆĪļÖŹµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ__________£»Š“³öÓĆøĆĪļÖŹÖĘ·ŹŌķµÄ»Æѧ·“Ó¦·½³ĢŹ½_________________________________________”£
£Ø2£©ŌŚÖĘ·ŹŌķŹ±¼ÓČėŅŅ“¼ŹĒĄūÓĆĮĖŅŅ“¼µÄŹ²Ć“ŠŌÖŹ£æ
_____________________________________________________________________________ӣ
£Ø3£©ŌŚ²Ł×÷¢ÜÖŠ¼ÓČė±„ŗĶNaClČÜŅŗµÄ×÷ÓĆŹĒ_____________________________________”£
£Ø4£©ĻĀĮŠ·“Ó¦ĄąŠĶĆū³ĘÖŠæɱķŹ¾£Ø1£©ÖŠ»Æѧ·“Ó¦ĄąŠĶµÄÓŠ________£ØĢīŠņŗÅ£©”£
A.¼Ó³É B.Č”“ś C.ĻūČ„ D.Ė®½ā E.Ńõ»Æ F.»¹Ō G.Ōķ»Æ H.õ„»Æ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com