
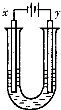
������X��ˮ��ҺΪdz��ɫ���ɷ�������ת����ϵ�����ַ�Ӧ��������ԣ�������B��D��E��F��Ϊ��ɫ���壬W��KΪ�����������ʣ�CΪ������ˮ�ĺ�ֹ��塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��W��Ӧ�ų��������ȡ�
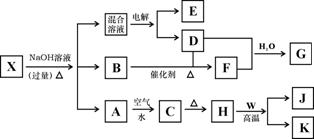
��ش��������⣺
��1��B����ļ��鷽��Ϊ
��2�������Һʱ����O2�ĵ缫��Ӧ����ʽ
��3����Ҫ��д������ת����ϵ�е��йط�Ӧ����ʽ
�ٺ���KԪ�صĻ��Ϸ�Ӧ
�ں���KԪ�ص��û���Ӧ
��4��K�����G��ϡ��Һ��Ӧ�����ӷ���ʽ
��5������X�Ļ�ѧʽ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| W | |||||||
| X | Y | Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ||
| ||
 Al��OH��3+OH-
Al��OH��3+OH- Al��OH��3+OH-
Al��OH��3+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��������ģ��A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶��С����Eͬ���壬BԪ��ԭ�ӵ��������������ڲ��������2����CԪ�ص�����������Ӧ��ˮ���������⻯�ﷴӦ��������X��D��E���γɵ���ɫ����Y��FԪ��ԭ�ӵ������������ȴ�����������1���밴Ҫ��ش��������⣺
��2012?��������ģ��A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶��С����Eͬ���壬BԪ��ԭ�ӵ��������������ڲ��������2����CԪ�ص�����������Ӧ��ˮ���������⻯�ﷴӦ��������X��D��E���γɵ���ɫ����Y��FԪ��ԭ�ӵ������������ȴ�����������1���밴Ҫ��ش��������⣺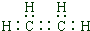
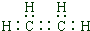
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ص���ѧЭ����2010������ڶ�����������ѧ�� ���ͣ������
������Ԫ��W��X��Y��Z����Ԫ�����ڱ��е�λ����ͼ��ʾ��
| W | | | | | | | |
| | | | X | Y | Z | | |
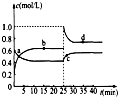
 Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��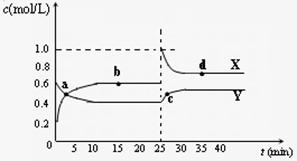 �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��W��X��Y��Z����Ԫ�����ڱ��е�λ����ͼ��ʾ��
| W | |||||||
| X | Y | Z |
����X��Y��Z����Ԫ�ص�������֮��Ϊ21��?
(1)W��Z�γ�ԭ�Ӹ�����Ϊ1��1�Ļ���������ʽΪ ��
(2)Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ���������ˮ��Һ��
���ԣ���ԭ���� (�û�ѧ�����ʾ)��
����Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
(3)��X W4��Z2��KOH��Һ��ɵ�����ȼ�ϵ���У������Ϸ�����Ӧ�ĵ缫��ӦʽΪ
��
(4)��֪��2YZ2(g) ![]() Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
t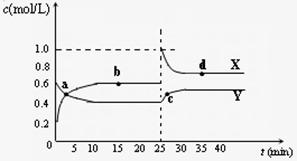 �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ��
�� a��b��c��d�ĸ����У���ѧ��Ӧ
����ƽ��״̬���� �㡣
�� 25 minʱ��������
�������ʵĻ�ѧʽ�� mol��
�� a��b��c��d�ĸ���������ʾ�ķ�Ӧ��ϵ
�У�������ɫ���dz��˳���� __
������ĸ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com