
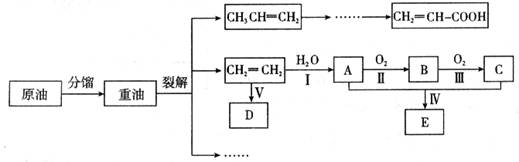
��10�֣���ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�
��֪��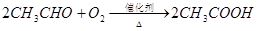
��1���������������������仯����__________������ţ���
�ٷ�����ѽ�
��2��A��������__________��
��3����ӦII�Ļ�ѧ����ʽ��__________��
��4��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ��__________��
��5��E������ζ�����ʣ���ʵ���ҿ�����ͼװ����ȡ��

�ٷ�ӦIV�Ļ�ѧ����ʽ��__________��
�ڳ��ڶ�ȫ�Ŀ��ǣ�ʵ����Ӧ�ò�ȡ�Ĵ�ʩ��__________����д��һ�ּ��ɣ�
��6�����й���CH2��CH��COOH��˵����ȷ����__________��
����CH3CH��CHCOOH��Ϊͬϵ��
�ڿ�����NaHCO3��Һ��Ӧ�ų�CO2����
����һ�������¿��Է���ȡ�����ӳɡ�������Ӧ
��1���٣�1�֣�
��2���ǻ���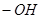 ��1�֣�
��1�֣�
��3��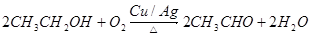 ��2�֣�����1�֣�Ҳ��д��
��2�֣�����1�֣�Ҳ��д�� ��
��
��4�� ��1�֣�
��1�֣�
��5����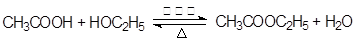 ��2�֣���Ũ���ᡢ���ȡ���
��2�֣���Ũ���ᡢ���ȡ��� ��ȱһ���1�֣�
��ȱһ���1�֣�
���Թܼ��м����ʯ��ֹ���л��Թ����е��ܿڲ�����Һ���·�ֹ�������Լ����ʱ���ȼ��Ҵ���Ȼ������Թܱ�������Ũ�������������ʱ���ȶ��Թܽ���Ԥ�ȣ�1�֣��δ�һ�㼴�ɣ�
��6���� �� �� ��2�֣����������1�֣�ֻ��һ�������֣�
����������1�����������÷е�IJ�ͬ�����з����һ�ַ����������������仯���ѽ��ǻ�ѧ�仯��
��2����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����A���Ҵ������������ǻ���
��3���Ҵ��ܷ�����������������ȩ����B����ȩ������ʽΪ
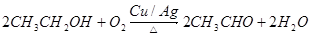 ��
��
��4����ϩ����̼̼˫�����ܷ����Ӿ۷�Ӧ���ɾ���ϩ���ṹ��ʽΪ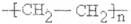 ��
��
��5������ȩ�����������ᣬ��C�����ᣬ������Ҵ�����������Ӧ������������������ʽΪ
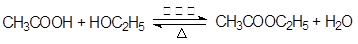 ��
��
��Һ�����������ڣ�����װ����Ҫ��ֹ�����������Լ�ʱ��Ҫ���ǵ�Ũ��������ԣ����Թܼ��м����ʯ��ֹ���л��Թ����е��ܿڲ�����Һ���·�ֹ�������Լ����ʱ���ȼ��Ҵ���Ȼ������Թܱ�������Ũ�������������ʱ���ȶ��Թܽ���Ԥ�ȵȡ�
��6���ж��л�������ʣ��ؼ����ҳ��л����к��еĹ����š������л���Ľṹ��ʽ��֪�������к��еĹ�������̼̼˫�����Ȼ�������ѡ��� �� �۶�����ȷ�ġ�


 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| �� |
| Cu��Ag |
| �� |
| Cu��Ag |
| �� |


| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱���г�������һ��ѧ����ĩͳһ���Ի�ѧ�Ծ����������� ���ͣ������
��10�֣���ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�
��֪��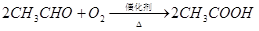
��1���������������������仯����__________������ţ���
�ٷ�����ѽ�
��2��A��������__________��
��3����ӦII�Ļ�ѧ����ʽ��__________��
��4��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ��__________��
��5��E������ζ�����ʣ���ʵ���ҿ�����ͼװ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ��__________��
�ڳ��ڶ�ȫ�Ŀ��ǣ�ʵ����Ӧ�ò�ȡ�Ĵ�ʩ��__________����д��һ�ּ��ɣ�
��6�����й���CH2��CH��COOH��˵����ȷ����__________��
����CH3CH��CHCOOH��Ϊͬϵ��
�ڿ�����NaHCO3��Һ��Ӧ�ų�CO2����
����һ�������¿��Է���ȡ�����ӳɡ�������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ�ij���������У��һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ʵ����
��ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�

��֪��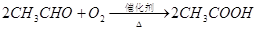
��1����ӦII�Ļ�ѧ����ʽ�� ��
��2��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ�� ��
��3��E������ζ�����ʣ���ʵ��������ͼװ����ȡ��
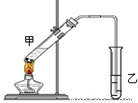
�ٷ�ӦIV�Ļ�ѧ����ʽ�� ���÷�Ӧ����Ϊ ��
�ڸ�װ��ͼ����һ�����ԵĴ����� ��
��4��Ϊ��֤��Ũ�����ڷ�ӦIV�����˴�������ˮ�������ã�ijͬѧ������ͼ�Ľ���װ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
ʵ���� �Թܼ����Լ� �Թ������Լ� �л���ĺ��/cm
A 2 mL�Ҵ���1 mL���ᡢ
1mL18mol��L��1 Ũ���� ����Na2CO3��Һ 3.0
B 2 mL�Ҵ���1 mL���� 0.1
C 2 mL�Ҵ���1 mL���ᡢ
3 mL 2mol��L��1 H2SO4 0.6
D 2 mL�Ҵ���1 mL���ᡢ���� 0.6
��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���3mL�� mol��L��1 ��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��8�֣���ѧ�����ߺ��������ʮ�ֹ�ע������ϩ����Ĥ�İ�ȫ���⡣��ҵ������ϩ������Ϊԭ�Ͼ������и�����Ӧ�ϳɾ�����ϩ��

��1����ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ�Ŀǰ��ҵ��������ϩ��Ҫ��ʯ��Ϊԭ��ͨ��__________��Ӧ��ʵ�֡�
��2����Ӧ�ٵĻ�ѧ����ʽΪ�� ��
��3���л���������Ȳ���ṹ�е�̼̼�����DZ�̼̼˫���������͵ļ����Ʋ���Ȳ���е����� ��д��һ�㼴�ɣ���
��4�����Ǿ�����ϩ�ĵ��壬��ṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com