
| ŹµŃ鱹ŗÅ | NaOHČÜŅŗĢå»ż£ØmL£© | “ż²āŃĪĖįµÄĢå»ż£ØmL£© |
| 1 | 22.62 | 20.00 |
| 2 | 22.72 | 20.00 |
| 3 | 22.80 | 20.00 |
·ÖĪö £Ø1£©ČēČÜŅŗŃÕÉ«±ä»ÆĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£¬æÉĖµĆ÷“ļµ½µĪ¶ØÖÕµć£»øł¾ŻÖøŹ¾¼ĮµÄ±äÉ«·¶Ī§Č·¶ØPH£»
£Ø2£©ĻČÅŠ¶ĻŹż¾ŻµÄŗĻĄķŠŌ£¬Ēó³ö±ź×¼NaOHČÜŅŗĢå»ż£¬Č»ŗóŅĄ¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$Ēó³ö¼“æÉ£»
£Ø3£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö²»µ±²Ł×÷¶ŌV£Ø±ź×¼£©µÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻÅØ¶ČµÄĪó²ī£»
½ā“š ½ā£ŗ£Ø1£©NaOHČÜŅŗµĪ¶ØŃĪĖį£¬·ÓĢŖ×÷ÖøŹ¾¼Į£¬µ±×īŗóŅ»µĪNaOHČÜŅŗ¼ÓČėŹ±£¬ČÜŅŗÓÉĪŽÉ«Ē”ŗƱä³ÉĒ³ŗģÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«ĪŖµĪ¶ØÖÕµć£¬·ÓĢŖµÄ±äÉ«·¶Ī§ŹĒ8.2”«10£¬ĖłŅŌµĪ¶ØÖÕµćŹĒČÜŅŗpHĪŖ8.2”«10£»
¹Ź“š°øĪŖ£ŗ×īŗóŅ»µĪĒāŃõ»ÆÄĘČÜŅŗ¼ÓČė£¬ČÜŅŗÓÉĪŽÉ«Ē”ŗƱä³ÉĒ³ŗģÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»8.2”«10£®
£Ø2£©ŹµŃé1Īó²ī½Ļ“ó£¬ÉįČ„£¬µĆ³öV£Ø±ź×¼£©=$\frac{22.72+22.80}{2}$=22.76mL£¬ĖłŅŌc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$=$\frac{0.1mol/L”Į22.76mL}{20.00mL}$=0.11mol/L£¬¹Ź“š°øĪŖ£ŗ0.11mol/L£»
£Ø3£©A£®µĪ¶ØÖÕµć¶ĮŹżŹ±ø©ŹÓ¶Į£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«Š”£¬¹ŹA“ķĪó£»
B£®ĖįŹ½µĪ¶Ø¹ÜŹ¹ÓĆĒ°£¬Ė®Ļ“ŗóĪ“ÓĆ“ż²āŃĪĖįČÜŅŗČóĻ“£¬“ż²āŅŗ±»Ļ”ŹĶ£¬ĘäĪļÖŹµÄĮæĘ«Š”£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«Š”£¬¹ŹB“ķĪó£»
C£®×¶ŠĪĘæĖ®Ļ“ŗóĪ“øÉŌļ£¬“ż²āŅŗµÄĪļÖŹµÄĮæ²»±ä£¬¶ŌV£Ø±ź×¼£©ĪŽÓ°Ļģ£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©²»±ä£¬¹ŹC“ķĪó£»
D£®1molNaOH¼“40gĻūŗÄ1molHCl£¬1molNa2CO3¼“106gĻūŗÄ2molHCl£¬ŌņµČÖŹĮæµÄNaOHŗĶNa2CO3ÓėŃĪĖį·“Ó¦£¬NaOHĻūŗĵÄŃĪĖį¶ą£¬ÓėµČĮæµÄŃĪĖį·“Ó¦Ź±£¬ŠčŅŖµÄ»ģÓŠNa2CO3µÄNaOH±ź×¼ČÜŅŗ½Ļ¶ą£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«“󣬹ŹDÕżČ·£»
E£®NaOH±ź×¼ČÜŅŗ²æ·ÖÓėæÕĘųÖŠµÄCO2·“Ӧɜ³ÉĮĖNa2CO3£¬øł¾ŻÄĘŹŲŗć£¬¶ŌV£Ø±ź×¼£©ĪŽÓ°Ļģ£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©²»±ä£¬¹ŹE“ķĪó£»
F£®¼īŹ½µĪ¶Ø¹Ü¼ā×ģ²æ·ÖÓŠĘųÅŻ£¬µĪ¶ØŗóĻūŹ§£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«“󣬹ŹFÕżČ·£»
¹ŹŃ”DF£®
µćĘĄ ±¾Ģāæ¼²éĮĖĖį¼īÖŠŗĶµĪ¶ØŹµŃé¼°Īó²ī·ÖĪö£¬ÕĘĪÕÖŠŗĶµĪ¶ØµÄ²Ł×÷·½·Ø¼°Ķź³É·ÖĪöµÄ·½·ØŹĒ½āĢā¹Ų¼ü£¬×¢ŅāŅĄ¾Ż¹«Ź½c£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$½ųŠŠĪó²ī·ÖĪö£¬ĢāÄæÄѶČÖŠµČ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģŗž±±Ź”¾£ĆÅŹŠøßČżÉĻ¾ÅŌĀĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
Ņ»ÖÖ»ŲŹÕ²¢ĄūÓĆŗ¬µā£ØI-£©·ĻŅŗµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ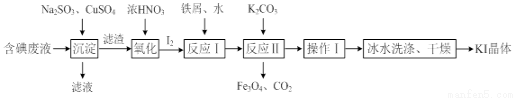
£Ø1£©”°³Įµķ”±ÖŠÉś³ÉCuIµÄĄė×Ó·½³ĢŹ½ĪŖ___________________”£
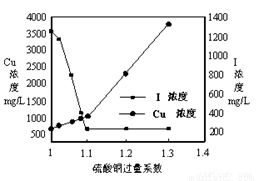
£Ø2£©CuSO4µÄĶ¶¼ÓĮæ¶Ō·ĻĖ®ÖŠI£µÄČ„³żĮæÓ°Ļģ½Ļ“󣬲»Ķ¬Ķ¶¼ÓĮæ£ØÓĆ¹żĮæĻµŹż±ķŹ¾£©ĻĀ£¬·“Ó¦ŗóI£ŗĶCu2+µÄÅضČČēĶ¼ĖłŹ¾£¬ŌņŹŹŅĖµÄCuSO4¹żĮæĻµŹżÓ¦ĪŖ_____________£¬·ÖĪöŌŅņ_________________”£
£Ø3£©·“Ó¦¢ńÖŠÉś³ÉĢśÓėµāµÄ»ÆŗĻĪļ£ØĘäÖŠĢśÓėµāµÄÖŹĮæ±ČĪŖ21£ŗ127£©£¬Ōņ¼ÓČėµÄĖ®µÄ×÷ÓĆŹĒ________________£¬·“Ó¦¢ņµÄ»Æѧ·½³ĢŹ½ŹĒ_________________________”£
£Ø4£©²Ł×÷¢ń°üĄØ____________£¬±łĖ®Ļ“µÓµÄÄæµÄŹĒ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2017½ģø£½ØŹ”øßČżÉĻµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
½«ĀČ»ÆĀĮČÜŅŗŗĶĒāŃõ»ÆÄĘČÜŅŗµČĢå»ż»ģŗĻ£¬µĆµ½µÄ³ĮµķĪļÖŠĀĮŌŖĖŲµÄÖŹĮæÓėČÜŅŗÖŠĖłŗ¬ĀĮŌŖĖŲµÄÖŹĮæĻąµČ£¬ŌņŌĀČ»ÆĀĮČÜŅŗŗĶĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅضČÖ®±ČæÉÄÜŹĒ( )
A£®1”Ć3 B£®2”Ć3 C£®1”Ć4 D£®2”Ć1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆÕōĮóĖ®Łž¾»µĪ¶Ø¹Üŗó£¬×°Čė±ź×¼ŃĪĖį½ųŠŠµĪ¶Ø | |
| B£® | ÓĆÕōĮóĖ®Łž¾»×¶ŠĪĘæŗó£¬ŌŁÓĆNaOHŅŗČóĻ“£¬¶ųŗó×°ČėŅ»¶ØĢå»żµÄNaOHČÜŅŗ | |
| C£® | ÓĆ¼īŹ½µĪ¶Ø¹ÜČ”10.00mLNaOHČÜŅŗ·ÅČėÓĆÕōĮóĖ®Ļ“¾»µÄ׶ŠĪĘæÖŠ£¬ŌŁ¼ÓČėŹŹĮæÕōĮóĖ®½ųŠŠµĪ¶Ø | |
| D£® | øÄÓĆŅĘŅŗ¹ÜČ”10.00mLNaOHČÜŅŗ£¬·ÅČė׶ŠĪĘæŗ󣬰ŃŅĘŅŗ¹Ü¼ā×ģ“¦ŅŗĢ哵Čė |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

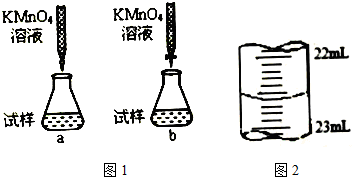
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | õ„»Æ·“Ó¦Ņ²ŹōÓŚ¼Ó³É·“Ó¦ | |
| B£® | õ„»Æ·“Ó¦ÖŠĖįĶŃČ„ōĒ»ł£¬“¼ĶŃČ„ĒāŌ×Ó | |
| C£® | ÅØĮņĖįŌŚõ„»Æ·“Ӧ֊ֻʚ“߻ƼĮµÄ×÷ÓĆ | |
| D£® | ŅŅĖįŅŅõ„µÄ½į¹¹¼ņŹ½ĪŖCH3COOCH3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŠšŹö | ŹµŃéĻÖĻó | ½āŹĶ |
| A | ·“ŻĶČ”·ØĢįČ”µāµÄCCl4ČÜŅŗÖŠµÄµā | Ļņø»µāµÄCCl4ČÜŅŗÖŠ¼ÓČė×ćĮæĖ®”¢Õńµ“”¢¾²ÖĆ”¢·ÖŅŗ | µāŌŚĖ®ÖŠČܽā¶Č±ČŌŚCCl4ÖŠ“ó |
| B | ŌŚFeCl3ČÜŅŗÖŠ¼ÓČėŹŹĮæFe·Ū³ä·Ö·“Ó¦ŗó£¬ŌŁµĪ¼Ó¼øµĪ»ĘÉ«ĢśĒč»Æ¼ŲČÜŅŗ | ²śÉśĄ¶É«³Įµķ | 2Fe3++FeØT3Fe2+ 3Fe2++2[Fe£ØCN£©6]3-ØTFe3[Fe£ØCN£©6]2”ż |
| C | Ļņ0.1mol/LµÄFe£ØNO3£©2ČÜŅŗÖŠµĪ¼ÓŃĪĖį | Õńµ“ŹŌ¹ÜŃÕÉ«¼ÓÉī | H+ŅÖÖĘFe2+Ė®½ā |
| D | ČƵ°°×ÖŹ“ÓĖ®ČÜŅŗÖŠĪö³ö | ½«CuSO4ČÜŅŗ¼ÓČėµ°°×ÖŹÖŠ·¢ÉśŃĪĪö | ÖŲ½šŹōĪŽ»śŃĪČÜŅŗæɽµµĶµ°°×ÖŹµÄČܽā¶Č |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
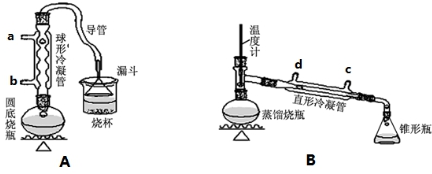
| ĪļÖŹ | ČŪµć/”ę | ·Šµć/”ę |
| 1-¶”“¼ | -89.5 | 117.3 |
| 1-ä嶔Ķé | -112.4 | 101.6 |
| ¶”ĆŃ | -95.3 | 142.4 |
| 1-¶”Ļ© | -185.3 | -6.5 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com