
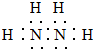 �����е��Ļ��ϼ�Ϊ-2��
�����е��Ļ��ϼ�Ϊ-2������ ��1���µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ����Ԫ�ػ��ϼ۴�����Ϊ0���㻯�ϼۣ�
��2������������������Һ���������£��������Ʊ���ԭ�����Ȼ��ƣ�
��3����2O2��g��+N2��g���TN2O4��l����H1
��N2��g��+2H2��g���TN2H4��l����H2
��O2��g��+2H2��g���T2H2O��g����H3
�����Ȼ�ѧ����ʽ��˹���ɼ���ۡ�2-�ڡ�2-�ٵõ���2N2H4��l��+N2O4��l���T3N2��g��+4H2O��g����H4=-1048.9kJ•mol-1
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ƣ�������һ�����뷽��ʽΪN2H4+H2O?N2H5++OH-��ƽ�ⳣ��Kb=$\frac{c��{N}_{2}{{H}_{5}}^{+}��c��O{H}^{-}��}{c��{N}_{2}{H}_{4}��}$=$\frac{c��{N}_{2}{{H}_{5}}^{+}��c��O{H}^{-}��}{c��{N}_{2}{H}_{4}��}$��$\frac{c��{H}^{+}��}{c��{H}^{+}��}$=K��Kw�������Ƕ�Ԫ���������������γɵ���ʽ��ΪN2H6��HSO4��2��
��5�������������������������ӱ���ԭ���ɵ�����������������ʧ����N2H4��N2-4e-��O2��4e-�������غ�����жϣ����ݹ�¯���ʵ��Լ���Ӧ�������ʽ��
��� �⣺��1���µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ��������ʽΪ��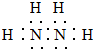 ��������Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�Ϊ-2�ۣ�
��������Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�Ϊ-2�ۣ�
�ʴ�Ϊ��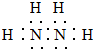 ��-2��
��-2��
��2������������������Һ���������£��������Ʊ���ԭ�����Ȼ��ƣ����ԭ���غ���ƽ��д��Ӧ�Ļ�ѧ����ʽΪ��2NH3+NaClO�TN2H4+NaCl+H2O��
�ʴ�Ϊ��2NH3+NaClO�TN2H4+NaCl+H2O��
��3����2O2��g��+N2��g���TN2O4��l����H1
��N2��g��+2H2��g���TN2H4��l����H2
��O2��g��+2H2��g���T2H2O��g����H3
�����Ȼ�ѧ����ʽ��˹���ɼ���ۡ�2-�ڡ�2-�ٵõ���2N2H4��l��+N2O4��l���T3N2��g��+4H2O��g����H4=2��H3-2��H2-��H1�����ݷ�Ӧ�ܿ�֪��������N2O4��Ӧ�ų��������Ҳ����������壬��˿���Ϊ����ƽ�����
�ʴ�Ϊ��2��H3-2��H2-��H1����Ӧ�����������������壻
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ƣ�������һ�����뷽��ʽΪN2H4+H2O?N2H5++OH-��ƽ�ⳣ��Kb=$\frac{c��{N}_{2}{{H}_{5}}^{+}��c��O{H}^{-}��}{c��{N}_{2}{H}_{4}��}$=$\frac{c��{N}_{2}{{H}_{5}}^{+}��c��O{H}^{-}��}{c��{N}_{2}{H}_{4}��}$��$\frac{c��{H}^{+}��}{c��{H}^{+}��}$=K��Kw=8.7��107��1.0��10-14=8.7��10-7���ڶ������뷽��ʽΪN2H5++H2O?N2H62++OH-����������������γɵ���ʽ��ΪN2H6��HSO4��2��
�ʴ�Ϊ��8.7��10-7��N2H6��HSO4��2��
��5�������������������������ӱ���ԭ���ɵ�������-2�۵�NԪ�ر�����ΪN2����Ӧ����ʽΪ��N2H4+4AgBr=4Ag��+N2��+4HBr����˷�Ӧ��������Ϊ��������ڣ��������ݲ����������µ����������ǵ���������Թ�¯��ɸ�ʴ�����������Ʊ���������Ϊ�����ƣ������������γ���Ӱ���¯�İ�ȫʹ�ã�����������ʧ����N2H4��N2ʧȥ4e-��O2��O2-�õ�4e-������������Ħ����������32g/mol����������������������ʵ�����ͬ��������1kg�������ɳ�ȥˮ���ܽ��O21kg����ʹ��Na2SO3����ˮ���ܽ��O2��ȣ��������ŵ��������٣��������������ʣ���Ӧ����ΪN2��H2O������Na2SO3����Na2SO4��
�ʴ�Ϊ��������ڣ��������ݲ�����1��N2H4�������٣��������������ʣ���Ӧ����ΪN2��H2O������Na2SO3����Na2SO4��
���� ���⿼���˵����仯�������ʡ����ʽṹ���Ȼ�ѧ����ʽ��˹���ɼ���Ӧ�á�ƽ�ⳣ���ļ��㷽������Ҫ��������ԭ��Ӧ�ļ��㼰�������жϣ���Ŀ�Ѷ��еȣ�


 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al����������չ�ԺͿ���ʴ�ԣ����Ƴ�������װ��Ʒ | |
| B�� | NH3 ����Cl2 ����NH4Cl������Ũ��ˮ�������������Ĺܵ��Ƿ���й© | |
| C�� | NaHCO3 ����Ӧ��ʳƷ��ҵ�������Ƹ������ɼ� | |
| D�� | K2FeO4 ����ˮ��������Fe��OH��3 �����O2�������ھ�������ˮ��ɱ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
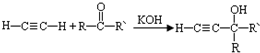
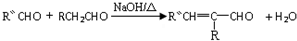 ��-R��-R�䡢-R���ʾ������ͬ����ܲ�ͬ��ԭ�ӻ�ԭ���ţ�
��-R��-R�䡢-R���ʾ������ͬ����ܲ�ͬ��ԭ�ӻ�ԭ���ţ� ��
�� ��
��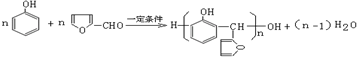 ��
��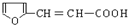 �ж���ͬ���칹�壬��������Ҫ�����9�֣�
�ж���ͬ���칹�壬��������Ҫ�����9�֣�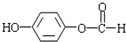 ��
��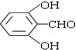 ��
��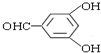 ����дһ�֣���
����дһ�֣����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��Na+��Fe2+��NO3- | B�� | Fe3+��Fe2+��SO42-��NO3- | ||
| C�� | Na+��OH-��SO42-��H2PO4- | D�� | Al3+��Na+��S2-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȼ��ȼ�ղ���CO2����������֮һ | |
| B�� | ��ʯȼ����ȫȼ�ղ�����ɴ�����Ⱦ | |
| C�� | ��Һ��ʯ��������ȼ�Ϳɼ��ٴ�����Ⱦ | |
| D�� | ȼ�ϲ���ȫȼ���ŷŵ�CO�Ǵ�����Ⱦ��֮һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
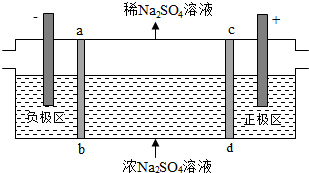
| A�� | ͨ����м���ҵ�SO42-����������Ǩ�ƣ���������ҺpH���� | |
| B�� | �÷��ڴ�����Na2SO4��ˮʱ���Եõ�NaOH��H2SO4��Ʒ | |
| C�� | ������ӦΪ2H2O-4e-=O2+4H+����������ҺpH���� | |
| D�� | ����·��ͨ��1mol���ӵĵ���ʱ������0.5mol��O2���� |
�鿴�𰸺ͽ���>>
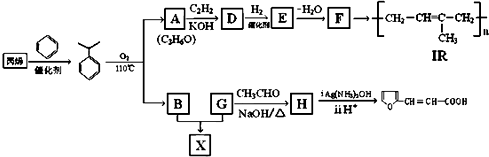
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
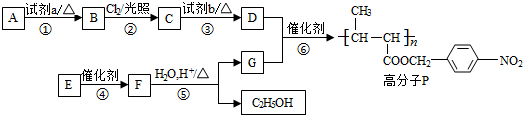
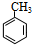 ��
�� +NaOH$��_{��}^{ˮ}$
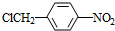
+NaOH$��_{��}^{ˮ}$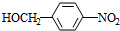 +NaCl��
+NaCl��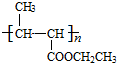 +nH2O$��_{��}^{H+}$
+nH2O$��_{��}^{H+}$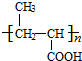 +n CH3CH2OH��
+n CH3CH2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7�� | B�� | 8�� | C�� | 9�� | D�� | 10�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��OH-��NO3- | B�� | Ba2+��H+��SO42- | C�� | K+��OH-��CO32- | D�� | Fe3+��Cl-��OH- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com