
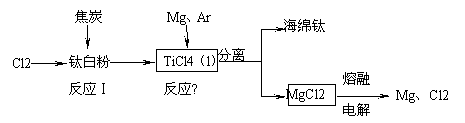
£Ø12·Ö£©21ŹĄ¼ĶŹĒīѵďĄ¼Ķ”£ĻĀĆęŹĒĄūÓĆīŃ°×·Ū£ØTiO2£©Éś²śŗ£ĆąīŃ£ØTi£©µÄŅ»ÖÖ¹¤ŅÕĮ÷³Ģ£ŗ
 |
ŅŃÖŖ£ŗ
¢Ł Mg(s)£«Cl2 (g)£½MgCl2 (s) ”÷H£½£641 kJ”¤mol£1
¢Ś Cl2(g)£«1/2Ti (s)£½1/2TiCl4 (l) ”÷H£½£385 kJ”¤mol£1
£Ø1£©īŃ°×·ŪŹĒĄūÓĆTiO2£«·¢ÉśĖ®½āÉś³ÉīŃĖį£ØH2TiO3£©³Įµķ£¬ŌŁģŃÉÕ³ĮµķÖʵƵĔ£TiO2£«·¢Éś
Ė®½āµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©·“Ó¦¢ńŌŚ800”«900”ęĻĀ½ųŠŠ£¬»¹Éś³ÉŅ»ÖÖæÉČ¼ŠŌĪŽÉ«ĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
 ĪŖ £»·“Ó¦¢ņµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
ĪŖ £»·“Ó¦¢ņµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©øĆ¹¤ŅÕĮ÷³ĢÖŠ£¬æÉŅŌŃ»·Ź¹ÓƵÄĪļÖŹÓŠ ”£
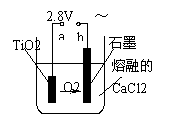
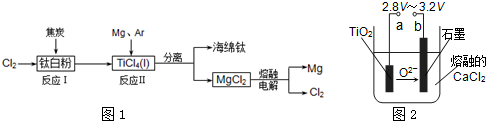
£Ø4£©ŌŚ800”ꔫ1000”ꏱµē½āTiO2Ņ²æÉÖʵĆŗ£ĆąīŃ£¬×°ÖĆČēÓŅ
Ķ¼ĖłŹ¾”£Ķ¼ÖŠbŹĒµēŌ“µÄ ¼«£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģĢģ½ņŹŠŹ®¶žŠ£øßČżµŚ¶ž“ĪÄ£ÄāĮŖæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
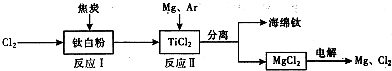
īŃ(Ti)±»³ĘĪŖ¼ĢĢś”¢ĀĮÖ®ŗóµÄµŚČż½šŹō£¬Ņ²ÓŠČĖĖµ21ŹĄ¼ĶŹĒīѵďĄ¼Ķ”£īŃŌŚµŲæĒÖŠµÄŗ¬Įæ²¢²»ÉŁ£¬µ«īѵÄŅ±Į¶¼¼Źõ»¹Ī“»ńµĆĶ»ĘĘ£¬ÄæĒ°īŃÖ»ÓĆÓŚ¼ā¶ĖĮģÓņ”£
ČēĻĀĶ¼ĖłŹ¾£¬½«īŃ³§”¢ĀČ¼ī³§ŗĶ¼×“¼³§×é³É²śŅµĮ“æÉ“ó“óĢįøß׏Ō“ĄūÓĆĀŹ£¬¼õÉŁ»·¾³ĪŪČ¾”£
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©ÓƶčŠŌµē¼«µē½ā2 LŹ³ŃĪĖ®Ź±£¬×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½_______________________________£¬µ±Ņõ¼«ÉĻ²śÉś224 mLĘųĢå£Ø±ź×¼×“æö£©Ź±£¬ĖłµĆČÜŅŗµÄpH= £Ø¼ŁÉčµē½āĒ°ŗóČÜŅŗĢå»ż²»±ä,Ź³ŃĪĖ®×ćĮ棩”£
£Ø2£©Š“³öøßĪĀĻĀīŃĢśæó¾ĀČ»ÆµĆµ½ĖÄĀČ»ÆīѵĻÆѧ·½³ĢŹ½ ”££ØĢįŹ¾£ŗFeTiO3ÖŠTiĪŖ+4¼Ū£©
£Ø3£©·“Ó¦2Mg£«TiCl4 2MgCl4£«TiŌŚArĘų·ÕÖŠ½ųŠŠµÄĄķÓÉŹĒ____________________”£
2MgCl4£«TiŌŚArĘų·ÕÖŠ½ųŠŠµÄĄķÓÉŹĒ____________________”£
£Ø4£©¶ž¼×ĆŃŹĒŅ»ÖÖÖŲŅŖµÄĒå½ąČ¼ĮĻ£¬æÉŅŌĶعż¼×“¼·Ö×Ó¼äĶŃĖ®ÖĘµĆ£ŗ
2CH3OH(g) CH3OCH3(g)+H2O(g)””¦¤H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)””¦¤H=" -23.5" kJ/mol
T1 ”ꏱ£¬ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ½ØĮ¢ÉĻŹöĘ½ŗā£¬ĢåĻµÖŠø÷×é·ÖÅضČĖꏱ¼ä±ä»ÆČēĻĀĶ¼ĖłŹ¾”£
¢ŁT1 ”ꏱ£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ””””””””””£»
¢ŚĻąĶ¬Ģõ¼žĻĀ£¬ČōøıäĘšŹ¼ÅØ¶Č£¬Ä³Ź±æĢø÷×é·ÖÅضČŅĄ“ĪĪŖc(CH3OH)="0.4" mol/L”¢c(H2O)="0.6" mol/L”¢(CH3OCH3)="1.2" mol/L£¬“ĖŹ±Õż”¢Äę·“Ó¦ĖŁĀŹµÄ“󊔣ŗvÕż””””””vÄę(Ģī”°>”±”¢”°<”±»ņ”°=”±)”£
£Ø5£©ŌŚÉĻŹö²śŅµĮ“ÖŠ£¬ŗĻ³É192¶Ö¼×“¼ĄķĀŪÉĻŠč¶īĶā²¹³äH2__________¶Ö £Ø²»æ¼ĀĒÉś²ś¹ż³ĢÖŠĪļÖŹµÄČĪŗĪĖšŹ§£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğĢģ½ņŹŠŹ®¶žŠ£øßČżµŚ¶ž“ĪÄ£ÄāĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
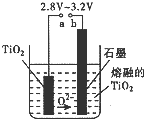
īŃ(Ti)±»³ĘĪŖ¼ĢĢś”¢ĀĮÖ®ŗóµÄµŚČż½šŹō£¬Ņ²ÓŠČĖĖµ21ŹĄ¼ĶŹĒīѵďĄ¼Ķ”£īŃŌŚµŲæĒÖŠµÄŗ¬Įæ²¢²»ÉŁ£¬µ«īѵÄŅ±Į¶¼¼Źõ»¹Ī“»ńµĆĶ»ĘĘ£¬ÄæĒ°īŃÖ»ÓĆÓŚ¼ā¶ĖĮģÓņ”£
ČēĻĀĶ¼ĖłŹ¾£¬½«īŃ³§”¢ĀČ¼ī³§ŗĶ¼×“¼³§×é³É²śŅµĮ“æÉ“ó“óĢįøß׏Ō“ĄūÓĆĀŹ£¬¼õÉŁ»·¾³ĪŪČ¾”£

ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©ÓƶčŠŌµē¼«µē½ā2 LŹ³ŃĪĖ®Ź±£¬×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½_______________________________£¬µ±Ņõ¼«ÉĻ²śÉś224 mLĘųĢå£Ø±ź×¼×“æö£©Ź±£¬ĖłµĆČÜŅŗµÄpH= £Ø¼ŁÉčµē½āĒ°ŗóČÜŅŗĢå»ż²»±ä,Ź³ŃĪĖ®×ćĮ棩”£
£Ø2£©Š“³öøßĪĀĻĀīŃĢśæó¾ĀČ»ÆµĆµ½ĖÄĀČ»ÆīѵĻÆѧ·½³ĢŹ½ ”££ØĢįŹ¾£ŗFeTiO3ÖŠTiĪŖ+4¼Ū£©
£Ø3£©·“Ó¦2Mg£«TiCl4 2MgCl4£«TiŌŚArĘų·ÕÖŠ½ųŠŠµÄĄķÓÉŹĒ____________________”£
2MgCl4£«TiŌŚArĘų·ÕÖŠ½ųŠŠµÄĄķÓÉŹĒ____________________”£
£Ø4£©¶ž¼×ĆŃŹĒŅ»ÖÖÖŲŅŖµÄĒå½ąČ¼ĮĻ£¬æÉŅŌĶعż¼×“¼·Ö×Ó¼äĶŃĖ®ÖĘµĆ£ŗ
2CH3OH(g) CH3OCH3(g)+H2O(g)””¦¤H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)””¦¤H=" -23.5" kJ/mol
T1 ”ꏱ£¬ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ½ØĮ¢ÉĻŹöĘ½ŗā£¬ĢåĻµÖŠø÷×é·ÖÅضČĖꏱ¼ä±ä»ÆČēĻĀĶ¼ĖłŹ¾”£

¢ŁT1 ”ꏱ£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ””””””””””£»
¢ŚĻąĶ¬Ģõ¼žĻĀ£¬ČōøıäĘšŹ¼ÅØ¶Č£¬Ä³Ź±æĢø÷×é·ÖÅضČŅĄ“ĪĪŖc(CH3OH)="0.4" mol/L”¢c(H2O)="0.6" mol/L”¢(CH3OCH3)="1.2" mol/L£¬“ĖŹ±Õż”¢Äę·“Ó¦ĖŁĀŹµÄ“󊔣ŗvÕż””””””vÄę(Ģī”°>”±”¢”°<”±»ņ”°=”±)”£
£Ø5£©ŌŚÉĻŹö²śŅµĮ“ÖŠ£¬ŗĻ³É192¶Ö¼×“¼ĄķĀŪÉĻŠč¶īĶā²¹³äH2__________¶Ö £Ø²»æ¼ĀĒÉś²ś¹ż³ĢÖŠĪļÖŹµÄČĪŗĪĖšŹ§£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com