
 ʵ����������0.05mol?L-1��NaOH��Һ500mL��������������
ʵ����������0.05mol?L-1��NaOH��Һ500mL��������������| n |
| V |


 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
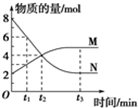 һ���¶��£���2L�ĺ����ܱ������ڷ����ķ�Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ��ʾ��
һ���¶��£���2L�ĺ����ܱ������ڷ����ķ�Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ѧ��Ӧ���ʱ仯ʱ����ѧƽ��һ�������ƶ� |
| B����ѧƽ�ⷢ���ƶ�ʱ����ѧ��Ӧ����һ���仯 |
| C������Ӧ���еij̶ȴ�����Ӧ����һ���� |
| D���ı�ѹǿ����ѧ��Ӧ����һ���ı䣬ƽ��һ���ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ο�����ͼ�����й�Ҫ��ش����⣺
�ο�����ͼ�����й�Ҫ��ش����⣺| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ܢ� |
| C���٢ۢ� | D���٢ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
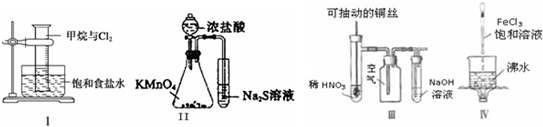
| A��ʵ��۲쵽��Ͳ�ڻ���ɫ����ʧ����Ͳ�ڱ�����״�������ɣ������������ڼ����������ڹ��������·������û���Ӧ |
| B��ʵ����ԱȽ�KMnO4��Cl2��S�����Ե����ǿ�� |
| C��ʵ�����ϡHNO3Ƭ�̣���Һ�������ݲ��������ƿ��ʼ�ձ�����ɫ |
| D��ʵ��������������Һ�����ɫ������ֹͣ���ȣ�������ͨ����ϵʱһ���ɲ��������ЧӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Һ���Ƿ���SO42-�����μ�����Լ���BaCl2��Һ��ϡ���� |
| B����pH=2����Һ�п��ܴ������У�Na+��AlO2-��CO32-��SO32- |
| C���ڳ����¼��������������ų�������Һ�п��ܴ������У�K+��Na+��OH-��NO3- |
| D��ʹ��ɫʯ����Һ����ɫ����Һ�п��ܴ������У�K+��Na+��Ca2+��HCO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com