
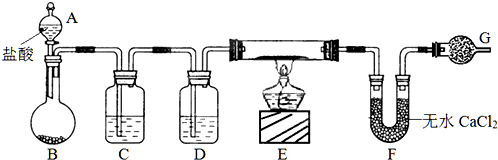
| ||
| 5(a-b) |
| m |
| ||
| 80n |
| 18m |
| 40n |
| 9m |
| 5(a-b) |
| m |
| 40n |
| 9m |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭCH3COOH��Һ��c��H+����NaOH��Һ��c��OH-����� |
| B���˹�����Һ��ˮ�ĵ���̶���������С����Һ��pH���� |
| C������VmLʱ����Һ������ |
| D������2VmLʱ����Һ��c��CH3COO-��+c��CH3COOH��=c��OH-��-c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
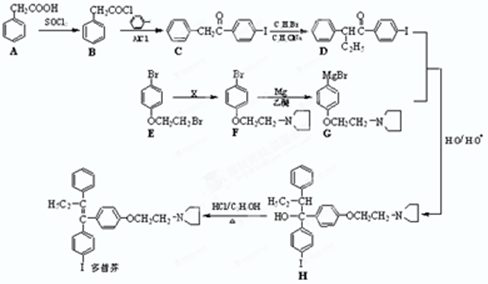
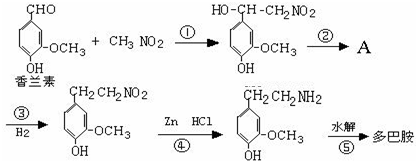
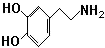
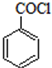 ���Ǻϳ�ҩƷ����Ҫ�м��壮��д���Ա������ѡ���ȩΪԭ���Ʊ��������ȵĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
���Ǻϳ�ҩƷ����Ҫ�м��壮��д���Ա������ѡ���ȩΪԭ���Ʊ��������ȵĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
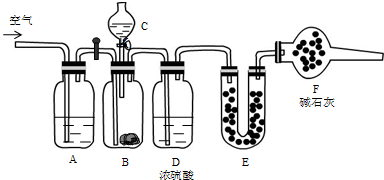
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| �� |
| һ������ |
| �� |
| ���¡���ѹ������ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
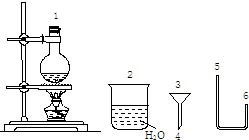 ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
������500mL0.2mol/LNa2CO3��Һ���ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com