
����Ŀ����ѧ��Ԥ��21������Ҷ�����������ܾ�����ʱ�����������ʶ��Ǿ��й���Ӧ��ǰ���Ĵ�����ϡ��ش��������⣺
(1)Zr(�)��Ԫ�����ڱ���λ�ڵ������ڣ�����ͬ�壬��̬Zr�ļ۲�����Ų�ʽΪ_______��
(2)�ǰ����(Li2NH) ����Ԫ�ص�һ��������С����____ ���縺��������_____ (��Ԫ�ط���)��
(3)����(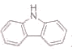 )�ķе����(
)�ķе����(![]() )�ߵ���Ҫԭ����________��
)�ߵ���Ҫԭ����________��
(4)��NH3BH3 (�����飬�۵�104��)�����黥Ϊ�ȵ����塣NH3BH3�ľ�������Ϊ____������B���ӻ�����Ϊ____����ͨ��_________�ⶨ�÷��ӵ����幹�͡�
��NH3BH3��ͨ�������顢CH4��H2O���кϳɣ����ǣ� CH4______H2O (����> "����<")��ԭ����________��
(5)MgH2�������ķ�Ʒϵ���ṹ��ͼ����������a =b= 450pm�� c= 30lpm��ԭ������ΪA(0��0��0)��B(0.305��0.305��0)��C(1��1��1)��D(0.195��0.805��0.5)��
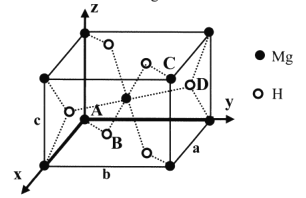
��Mg2+�İ뾶Ϊ72pm����H+�İ뾶Ϊ______pm (�г��������ʽ)
����NA��ʾ�����ӵ�������MgH2����������ܶ��DZ�״���������ܶȵ�_____��(�г��������ʽ�������ܶ�Ϊ0.089g��L-1)��
���𰸡�4d25s2 Li N ������Ӽ������� ���Ӿ��� sp3 ������� �� CH4���µ��Ӷԣ�H2O�������Թµ��Ӷԣ��µ��Ӷ�������֮����ų��������� ��֮����ų�����ʹ��CH4�еļ��DZ�H2O �еļ��Ǵ� ![]()
![]() ��
�� ![]()
��������
��1��Zr(�)��Ԫ�����ڱ���λ�ڵ������ڣ�����ͬ�壬���ڢ�B�壬���̬Zr�ļ۲�����Ų�ʽΪ��
��2��Li��H����ͬ���壬��һ�������ɴ��С��H>Li���縺����С���H<Li ��Li��N����ͬ���ڣ���һ��������С���Li<N�� �縺����С���Li<N��
��3����������ӽṹ��ʽ��֪����������Ӽ����N��H��������۷е�ϸߣ�
��4����NH3BH3�۵�ϵͣ���NH3BH3Ϊ���Ӿ��壻��������ԭ�Ӽ۲���ӶԻ��������ж�B���ӻ����ͣ���ͨ��������ײⶨ�÷��ӵ����幹�ͣ�
��5�������ÿռ伸�μ���AB�ľ���=Mg2+�İ뾶+H+�İ뾶�������þ�̯�����㾧�������ܶȡ�
��1��Zr(�)��Ԫ�����ڱ���λ�ڵ������ڣ�����ͬ�壬���ڢ�B�壬���ݺ�������Ų�����д����̬Zr�ļ۲�����Ų�ʽΪ4d25s2���ʴ�Ϊ��4d25s2��
��2��Li��H����ͬ���壬��һ�������ɴ��С��H>Li���縺����С���H<Li ��Li��N����ͬ���ڣ���һ��������С���Li<N�� �縺����С���Li<N���ʴ�Ϊ��Li��N��
��3����������ӽṹ��ʽ��֪����������Ӽ����N��H��������۷е�ϸߣ��ʴ�Ϊ��������Ӽ���������
��4����NH3BH3�۵�ϵͣ���NH3BH3Ϊ���Ӿ��壻NH3BH3��Bԭ�Ӻ���3���Ҽ���1�Թµ��Ӷԣ����ӻ�����Ϊsp3�ӻ�����ͨ��������ײⶨ�÷��ӵ����幹�ͣ��ʴ�Ϊ��sp3��������ף�
������ԭ�ӹµ��Ӷ�Խ�����ԽС��CH4��H2O������sp3�ӻ���CH4���µ��Ӷԣ�H2O�������Թµ��Ӷԣ��µ��Ӷ�������֮����ų��������� ��֮����ų�����ʹ��CH4�еļ��DZ�H2O �еļ��Ǵʴ�Ϊ������CH4���µ��Ӷԣ�H2O�������Թµ��Ӷԣ��µ��Ӷ�������֮����ų��������� ��֮����ų�����ʹ��CH4�еļ��DZ�H2O�еļ��Ǵ�
��5�������ÿռ伸�μ���AB�ľ���=![]() =Mg2+�İ뾶+H+�İ뾶����H+�İ뾶=
=Mg2+�İ뾶+H+�İ뾶����H+�İ뾶=![]() ���ھ�����H+����Ϊ
���ھ�����H+����Ϊ![]() ����������ܶ�
����������ܶ�![]()
![]() ��
�� ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
�� ![]() ��
�� ![]() ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ����٤��������ֵ�������ж���ȷ����( )
A.46g��NO2��N2O4�Ļ��������ԭ������Ϊ3NA
B.24g Mg��ΪMg2+ʱʧȥ�ĵ�����ĿΪNA
C.1mol/L CaCl2��Һ�к��е�Cl-������ĿΪ2NA
D.����lmol FeCl3����Һ��������ȫת��Ϊ�����������壬���н�������ĿΪNA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������,�� 20 mL 0.2 mol/LH2A��Һ�еμ�0. 2 mol/L NaOH��Һ.�й��������ʵ����仯������ͼ��ʾ(����I����H2A.��II����HA����III��A2��)������ͼʾ.�ж�����˵����ȷ����

A.��V(NaOH)=20 mLʱ.��Һ�и�����Ũ�ȵĴ�С��ϵΪc(Na+)>c(HA��)>c(H+)>c(A2һ)>c(OH��)
B.������������ʵ���Ũ�ȵ�NaOH��Һ��H2 A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�еĴ�
C.NaHA��Һ��:c(OH��)+2c(A2��) =c(H+) +c(H2A )
D.��Na2A��Һ��ˮϡ��.��Һ���������ӵ�Ũ�ȶ���С.���������ӵ����ʵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ѡ�95%�Ҵ����ݶ��ٸ�Ҷ���õ���ȡҺ�����������ԭ��ֲ�Ʒ���ֹ����������£�����˵���������
A.�����£���ԭ��������ˮ
B.�������Ҫ�ɷ�����ԭ��
C.��ѹ�����Ŀ���ǽ��������¶ȣ�������ԭ�����
D.��ԭ��ֲ�Ʒ����ͨ���ؽᾧ��һ���ᴿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CoS2��CO����������й����Ĺ�ҵ��ǰ�����ش��������⣺
(1)��֪��
CoS2(s) +CO(g) ![]() CoS(s) +COS(g) H1
CoS(s) +COS(g) H1
2COS(g) +SO2(g) ![]() 3S(s) +2CO2(g) H2
3S(s) +2CO2(g) H2
S(s) +CoS(S) ![]() CoS2 (s) ��H3
CoS2 (s) ��H3
��2CO(g)+ SO2(g)![]() 2CO2(g)+S(s) H4=____�� (��H1�� H2��H3��ʾ)
2CO2(g)+S(s) H4=____�� (��H1�� H2��H3��ʾ)
(2)�ں��¡���ѹ��������ģ���������SO2��ʼ����Ϊ1mol�����CO2��ƽ�����������CO��SO2��Ͷ�ϱȱ仯��ͼ��

�ٵ�Ͷ�ϱ�Ϊ2ʱ��t min ʱ���SO2ת����Ϊ50%������S���������ʱ�ʾ�ķ�Ӧ����v=______g��min-1��
�ڵ�Ͷ�ϱ�Ϊ3ʱ��CO2 ��ƽ�����������Ӧ�ĵ���______________��
(3)�������Ϊ1L�ĺ��¡������������ͨ��2 mol CO��| mol SO2����Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��
�������I��II�ı�����������____________________��
��SO2��ƽ��ת����Ϊ______��ƽ�ⳣ��Kp =________(��ƽ���ѹ����ƽ��Ũ�ȼ���)��
(4)���õ�ⷨ����SO2β�����Ʊ����շ� (Na2S2O4).���װ����ͼ����a____ b (����>�� ��=������<��)������S2O42-�ĵ缫��ӦʽΪ____________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����
��ѧ��ȤС���ijƷ�������е�Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ƣ�����������ɣ������������ɷ���������ʱ���������.
I.Ħ���������������Ķ��Լ��飺ȡ����������Ʒ����ˮ������裬����.
(1)�������м������NaOH��Һ������. ����������NaOH��Һ��Ӧ�����ӷ���ʽ��_______.
(2)��(1)������Һ����ͨ�����������̼���ټ������ϡ����.��һ���̷�����Ӧ�Ļ�ѧ����ʽ����Ϊ��__________________________________��__________________________________.
II.������Ʒ��̼��ƵĶ����ⶨ��������ͼ��ʾװ��(ͼ�мг�������ȥ)����ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ���������.

����ʵ����̻ش��������⣺
(3)ʵ����������������ͨ�����.�����ó��˿ɽ���B��C�еķ�Ӧ���⣬���У�_________.
(4)C�з�Ӧ����BaCO3�����ӷ���ʽ��___________________________________.
(5)���и����ʩ�У�������߲ⶨȷ�ȵ�����______��(����).
A.�ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B.�μ�����˹���
C.��A��B֮������ʢ��Ũ�����ϴ��װ��
D.��B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
(6)ʵ����ȷ��ȡ8.00 g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94 g.����Ʒ��̼��Ƶ���������Ϊ________.
(7)������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ���������.ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����_________________.
(8)װ����U�ι�D�еļ�ʯ�ҵ�������_____________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ǿ��淴ӦA+2B ![]() 2C + 3D �Ļ�ѧ��Ӧ�����뻯ѧƽ������������ı���仯��������ɴ��ƶϴ������
2C + 3D �Ļ�ѧ��Ӧ�����뻯ѧƽ������������ı���仯��������ɴ��ƶϴ������

A.A��Bһ��������
B.C����������
C.Dһ����������
D.����Ӧ�Ƿ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼���ƾ��壨Na2CO3��10H2O����Է�������286��������0.1mol/L��̼������Һ980mL����������������ȷ�����������������������Һ��Ũ��ƫ�ߵ���
A. ��ȡ̼���ƾ���28.6g
B. �ܽ�ʱ���м��ȣ���������Һת�Ƶ�����ƿ�����̶���
C. ת��ʱ���������ܽ�̼���ƾ�����ձ�û�н���ϴ��
D. ���ݺ�����ƿ��ҡ�ȣ����÷���Һ����ڿ̶��ߣ��ּ�������ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ�����·�Ӧ�ɻ��������Դ������(![]() )������˵������ȷ����
)������˵������ȷ����
��![]()
![]()
��![]()
![]()
��![]()
![]()
��![]()
![]()
A. ��Ӧ�١���Ϊ��Ӧ���ṩԭ����
B. ��Ӧ��Ҳ��![]() ��Դ�����õķ���֮һ
��Դ�����õķ���֮һ
C. ��Ӧ![]() ��
��![]()
D. ��Ӧ![]() ��
��![]()
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com