
(8��) ij�л���A����Է�������Ϊ62��Ϊ��һ���ⶨA�Ļ�ѧʽ����ȡ6.2 gA��ȫȼ�գ��õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����
5.4 g��8.8 g (����ÿ����Ӧ��ȫ)��
��1�����л����ʵ��ʽ�� ������ʽ�� ��
��2�����������ʾ�С�C��C�����͡�OһH�����������գ����˴�����������2�����շ壬�����֮��Ϊ1��2���ƶϸ��л���Ľṹ��ʽ�� ��
��3�����л���������Ʒ�Ӧ�Ļ�ѧ����ʽ�� ��
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
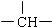 �ͱ�������ṹ��ʽΪ
�ͱ�������ṹ��ʽΪ

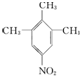 ��
��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
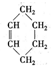

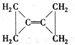

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ������
�����8�֣�
ij�л���A����NaOH��Һ��Ӧ��������к��б�������Է�������С��150�����к�̼����������Ϊ70.6%�������������Ϊ5.9%������Ϊ����
��1��A�ķ���ʽ�� ��
��2����A����NaHCO3��Һ��Ӧ�ų�CO2���壬��ṹ������ �֡�
��3����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1molA����1mol NaOH����A�����п��ܵĽṹ��ʽ�� ��
��4����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1mol A����2mol NaOH�������������A�Ľṹ������ �֣����в��ܷ���������Ӧ�����ʵĽṹ��ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����У�Ϻ��У�����������ѧ�Ծ��������棩 ���ͣ������
�����8�֣�
ij�л���A����NaOH��Һ��Ӧ��������к��б�������Է�������С��150�����к�̼����������Ϊ70.6%�������������Ϊ5.9%������Ϊ����
��1��A�ķ���ʽ�� ��
��2����A����NaHCO3��Һ��Ӧ�ų�CO2���壬��ṹ������ �֡�
��3����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1molA����1mol NaOH����A�����п��ܵĽṹ��ʽ�� ��
��4����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1mol A����2mol NaOH�������������A�Ľṹ������ �֣����в��ܷ���������Ӧ�����ʵĽṹ��ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com