
| ���� |
| �� |

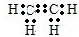 ���ʴ�Ϊ��
���ʴ�Ϊ��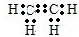 ��
��| ���� |
| Cu |
| �� |
| ���� |
| Cu |
| �� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��H2O��D2O��ͬλ�� |
| B����������Ԫ��ֻ�����ˮ |
| C��ˮ����֮�䲻ֻ���ڷ��»��� |
| D��ͼ�����ģ��Ҳ�ɱ�ʾCO2�Ľṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
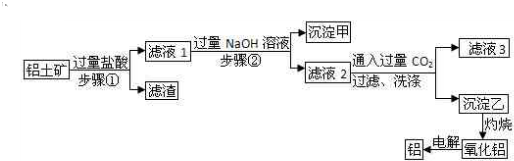
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
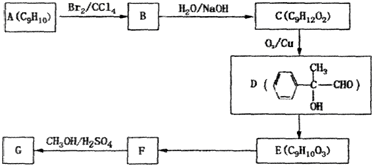
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| W | X | ||
| Y | Z |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Na2SO4��Һ���뵽����ʯ��ˮ�У��а�ɫ����������˵��Ksp[Ca��OH��2]����Ksp[CaSO4] |
| B��Kspֻ�����ܵ���ʵ����ʺ��¶��йأ�������Һ�е�����Ũ���� |
| C����֪25��ʱ��Ksp[Fe��OH��3]=4.0��10-38�����¶��·�ӦFe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ��K=4.0��104 |
| D����֪25��ʱ��Ksp[Mg��OH��2]=1.8��10-11����MgCl2��Һ�м��백ˮ����û��Һ��pH=11������Һ�е�c��Mg2+��Ϊ1.8��10-3mol��L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Ӧ�����У�c��H+��+c��Na+��+c��NH4+��=c��OH-��+2c��SO42-�� |
| B��������30mL NaOH��Һʱ��pH��7����c��NH4+����c��NH3?H2O����c��OH-����c��H+�� |
| C��������20mL NaOH��Һʱ��2c��SO42-��=c��NH3?H2O��+c��NH4+�� |
| D������Һ������ʱ��c��NH4+����c��SO42-����c��Na+����c��H+��=c��OH-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com