£Ø2012?³¤“ŗČżÄ££©ĮņĖį³§ÓĆģŃÉÕ»ĘĢśæó£ØFeS
2£©Ą“ÖĘČ”ĮņĖį£¬ŹµŃéŹŅĄūÓĆĮņĖį³§ÉÕŌü£ØÖ÷ŅŖ³É·ÖŹĒFe
2O
3¼°ÉŁĮæFeS”¢SiO
2£©ÖʱøĀĢ·Æ£®
£Ø1£©SO
2ŗĶO
2·“Ó¦ÖĘČ”SO
3µÄ·“Ó¦ŌĄķĪŖ£ŗ2SO
2+O
22SO
3£¬ŌŚŅ»ĆܱÕČŻĘ÷ÖŠŅ»¶ØŹ±¼äÄŚ“ļµ½Ę½ŗā£®
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£ŗ
£®
¢ŚøĆ·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄ±źÖ¾ŹĒ
BD
BD
£®
A£®v£ØSO
2£©=v£ØSO
3£© B£®»ģŗĻĪļµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»±ä
C£®»ģŗĻĘųĢåÖŹĮæ²»±ä D£®ø÷×é·ÖµÄĢå»ż·ÖŹż²»±ä
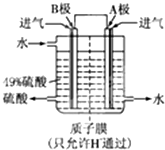
£Ø2£©Ä³æĘŃŠµ„Ī»ĄūÓĆŌµē³ŲŌĄķ£¬ÓĆSO
2ŗĶO
2Ą“ÖʱøĮņĖį£¬×°ÖĆČēĶ¼£¬µē¼«ĪŖ¶ąæ׵IJÄĮĻ£¬ÄÜĪüø½ĘųĢ壬Ķ¬Ź±Ņ²ÄÜŹ¹ĘųĢåÓėµē½āÖŹČÜŅŗ³ä·Ö½Ó“„£®
¢ŁBµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ
SO2-2e-+2H2OØTSO42-+4H+
SO2-2e-+2H2OØTSO42-+4H+
£»
¢ŚČÜŅŗÖŠH
+µÄŅĘ¶Æ·½ĻņÓÉ
B
B
¼«µ½
A
A
¼«£»
µē³Ų×Ü·“Ó¦Ź½ĪŖ
2SO2+O2+2H2OØT2H2SO4
2SO2+O2+2H2OØT2H2SO4
£®
£Ø3£©ĄūÓĆÉÕŌüÖĘĀĢ·ÆµÄ¹ż³ĢČēĻĀ£ŗ
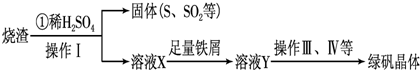
²ā¶ØĀĢ·Æ²śĘ·ÖŠŗ¬ĮæµÄŹµŃé²½Öč£ŗ
a£®³ĘČ”5.7g²śĘ·£¬Čܽā£¬Åä³É250mLČÜŅŗ
b£®ĮæČ”25mL“ż²āŅŗӌ׶ŠĪĘæÖŠ
c£®ÓĆĮņĖįĖį»ÆµÄ0.01mol/L KMnO
4ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄKMnO
4ČÜŅŗĢå»ż40mL
øł¾ŻÉĻŹö²½Öč»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁµĪ¶ØŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ØĶź³É²¢ÅäĘ½Ąė×Ó·“Ó¦·½³ĢŹ½£©£®
5
5
Fe
2++
1
1
Mn+
8
8
H+
H+
--
5
5
Fe
3++
1
1
Mn
2++
4
4
H2O
H2O
¢ŚÓĆĮņĖįĖį»ÆµÄKMnO
4µĪ¶ØÖÕµćµÄ±źÖ¾ŹĒ
µĪ¶Ø×īŗóŅ»µĪĖįŠŌKMnO4Ź±ČÜŅŗ³Źµ×ĻÉ«£¬°ė·ÖÖÓÄŚ²»ĶŹÉ«
µĪ¶Ø×īŗóŅ»µĪĖįŠŌKMnO4Ź±ČÜŅŗ³Źµ×ĻÉ«£¬°ė·ÖÖÓÄŚ²»ĶŹÉ«
£®
¢Ū¼ĘĖćÉĻŹö²śĘ·ÖŠFeSO
4?7H
2OµÄÖŹĮæ·ÖŹżĪŖ
0.975»ņ97.5%
0.975»ņ97.5%
£®

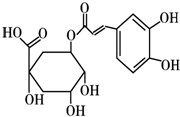 £Ø2012?³¤“ŗČżÄ££©[»Æѧ-Ń”ŠŽ ÓŠ»ś»Æѧ»ł“”]×¢ÉäÓĆĖ«»ĘĮ“ŅŌĀĢŌĖįĪŖÖ÷ŅŖ³É·Ö£ØĘä½į¹¹ČēĶ¼£©ĀĢŌĖįÓŠ¹ć·ŗµÄŅ©Ąķ×÷ÓĆ£®
£Ø2012?³¤“ŗČżÄ££©[»Æѧ-Ń”ŠŽ ÓŠ»ś»Æѧ»ł“”]×¢ÉäÓĆĖ«»ĘĮ“ŅŌĀĢŌĖįĪŖÖ÷ŅŖ³É·Ö£ØĘä½į¹¹ČēĶ¼£©ĀĢŌĖįÓŠ¹ć·ŗµÄŅ©Ąķ×÷ÓĆ£®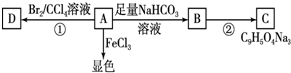
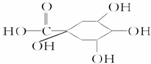


 £¬æ§·ČĖįAµÄ½į¹¹ĪŖ
£¬æ§·ČĖįAµÄ½į¹¹ĪŖ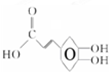 £¬æ§·ČĖįA·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¹ŹÓėBr2/CC14ČÜŅŗ·“Ó¦ĪŖ¼Ó³É·“Ó¦£®æ§·ČĖį
£¬æ§·ČĖįA·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¹ŹÓėBr2/CC14ČÜŅŗ·“Ó¦ĪŖ¼Ó³É·“Ó¦£®æ§·ČĖį ŗ¬ÓŠōČ»łŗĶ·ÓōĒ»ł£¬·ÓōĒ»łŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹA
ŗ¬ÓŠōČ»łŗĶ·ÓōĒ»ł£¬·ÓōĒ»łŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹA ÓėĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉµÄBĪŖ
ÓėĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉµÄBĪŖ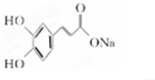 £¬BÖŠŗ¬ÓŠ·ÓōĒ»łÄÜÓėÓėĒāŃõ»ÆÄĘ·“Ó¦£¬Éś³ÉµÄCĪŖ
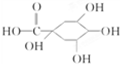
£¬BÖŠŗ¬ÓŠ·ÓōĒ»łÄÜÓėÓėĒāŃõ»ÆÄĘ·“Ó¦£¬Éś³ÉµÄCĪŖ £¬»ÆѧŹ½ĪŖC9H5O4Na3£¬¾Ż“ĖæÉĶĘ£®
£¬»ÆѧŹ½ĪŖC9H5O4Na3£¬¾Ż“ĖæÉĶĘ£® £¬æ§·ČĖįAµÄ½į¹¹ĪŖ
£¬æ§·ČĖįAµÄ½į¹¹ĪŖ £¬æ§·ČĖįA·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¹ŹÓėBr2/CC14ČÜŅŗ·“Ó¦ĪŖ¼Ó³É·“Ó¦£®æ§·ČĖį
£¬æ§·ČĖįA·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¹ŹÓėBr2/CC14ČÜŅŗ·“Ó¦ĪŖ¼Ó³É·“Ó¦£®æ§·ČĖį ŗ¬ÓŠōČ»łŗĶ·ÓōĒ»ł£¬·ÓōĒ»łŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹA
ŗ¬ÓŠōČ»łŗĶ·ÓōĒ»ł£¬·ÓōĒ»łŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹA ÓėĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉµÄBĪŖ
ÓėĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉµÄBĪŖ £¬BÖŠŗ¬ÓŠ·ÓōĒ»łÄÜÓėÓėĒāŃõ»ÆÄĘ·“Ó¦£¬Éś³ÉµÄCĪŖ
£¬BÖŠŗ¬ÓŠ·ÓōĒ»łÄÜÓėÓėĒāŃõ»ÆÄĘ·“Ó¦£¬Éś³ÉµÄCĪŖ £¬»ÆѧŹ½ĪŖC9H5O4Na3£¬
£¬»ÆѧŹ½ĪŖC9H5O4Na3£¬ £¬
£¬ £»
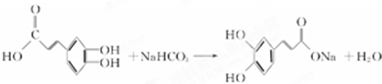
£» £¬æ§·ČĖį·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¹ŹÓėBr2/CC14ČÜŅŗ·“Ó¦ĪŖ¼Ó³É·“Ó¦£¬¹Ź“š°øĪŖ£ŗ¼Ó³É·“Ó¦£»
£¬æ§·ČĖį·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬¹ŹÓėBr2/CC14ČÜŅŗ·“Ó¦ĪŖ¼Ó³É·“Ó¦£¬¹Ź“š°øĪŖ£ŗ¼Ó³É·“Ó¦£» £¬B·Ö×ÓÖŠŗ¬ÓŠĮ½øö·ÓōĒ»ł¹ŹÓėĒāŃõ»ÆÄĘ1£ŗ2·“Ó¦£¬Ņņ“Ė1mol B
£¬B·Ö×ÓÖŠŗ¬ÓŠĮ½øö·ÓōĒ»ł¹ŹÓėĒāŃõ»ÆÄĘ1£ŗ2·“Ó¦£¬Ņņ“Ė1mol B Éś³É1mol C
Éś³É1mol C ŠčĒāŃõ»ÆÄĘ2mol£¬
ŠčĒāŃõ»ÆÄĘ2mol£¬ ŗ¬ÓŠōČ»łŗĶ·ÓōĒ»ł£¬·ÓōĒ»łŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹA
ŗ¬ÓŠōČ»łŗĶ·ÓōĒ»ł£¬·ÓōĒ»łŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹA ÓėĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉµÄBĪŖ
ÓėĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉµÄBĪŖ £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬
£¬ £»
£»


 £Ø2012?³¤“ŗČżÄ££©A”¢B”¢C”¢D¶¼ŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹ£¬ĘäÖŠA”¢B”¢C¾łŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬ŌŚŅ»¶ØĢõ¼žĻĀĻą»„×Ŗ»Æ¹ŲĻµČēĶ¼£Ø²æ·Ö·“Ó¦ÖŠµÄĖ®ŅŃĀŌČ„£©£®
£Ø2012?³¤“ŗČżÄ££©A”¢B”¢C”¢D¶¼ŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹ£¬ĘäÖŠA”¢B”¢C¾łŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬ŌŚŅ»¶ØĢõ¼žĻĀĻą»„×Ŗ»Æ¹ŲĻµČēĶ¼£Ø²æ·Ö·“Ó¦ÖŠµÄĖ®ŅŃĀŌČ„£©£®
