
��6�֣��ֽ�0.4molA�����0.2molB�������10L ���ܱ������У���һ��������ʹ�䷢����Ӧ��������C�������ʵ���  �ı仯����ͼ��
�ı仯����ͼ��
��1����t1=10min����0��t1ʱ����C���ʵ�ƽ����Ӧ����Ϊ ���÷�Ӧ��t2ʱ�̴ﵽƽ�⣬���仯ѧ��Ӧ����ʽΪ ��
��2����ͼ�����߱仯���������t1ʱ�̸ı�ķ�Ӧ���������� ��
A�������˴��� B�������˷�Ӧ�¶�
C�������������C D�������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�������ʡ���������и������ĴΣ�12�£��¿���ѧ�Ծ����������� ���ͣ������
��11�֣���ҵ���������Ҫ��Ӧ�ǣ�4NH3(g)+5O2(g) 4NO(g)+6H2O (g) ��H=" -1025" KJ/mol
4NO(g)+6H2O (g) ��H=" -1025" KJ/mol
��1��һ���¶��£��ֽ�0.8mol NH3��1.5mol O2����һ��ѹ�ܱ������У�����ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬���� 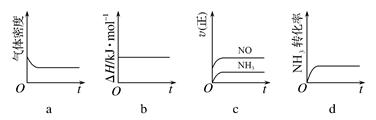
��2�����ݻ�Ϊ1L���ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
| ʱ�䣯Ũ�� | c(NH3)(mol/L) | C(O2)(mol/L) | C(NO)(mol/L) |
| ��ʼ | 0.8 | 1.5 | 0 |
| ��2min | 0.7 | a | 0.1 |
| ��4min | 0.4 | 1.0 | 0.4 |
| ��6min | 0.4 | 1.0 | 0.4 |
| ��8min | 1.2 | 2.5 | 0.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ�������ĴΣ�12�£��¿���ѧ�Ծ��������棩 ���ͣ������
��11�֣���ҵ���������Ҫ��Ӧ�ǣ�4NH3(g)+5O2(g) 4NO(g)+6H2O (g) ��H="
-1025" KJ/mol
4NO(g)+6H2O (g) ��H="
-1025" KJ/mol
��1��һ���¶��£��ֽ�0.8mol NH3��1.5mol O2����һ��ѹ�ܱ������У�����ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬����
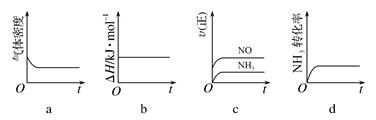
��2�����ݻ�Ϊ1L���ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
|
ʱ�䣯Ũ�� |
c(NH3)(mol/L) |
C(O2)(mol/L) |
C(NO)(mol/L) |
|
��ʼ |
0.8 |
1.5 |
0 |
|
��2min |
0.7 |
a |
0.1 |
|
��4min |
0.4 |
1.0 |
0.4 |
|
��6min |
0.4 |
1.0 |
0.4 |
|
��8min |
1.2 |
2.5 |
0.4 |
����ʼʱ���ϱ�c (O2) : c (NH3)��1.25����ԭ����
�ڷ�Ӧ�ڵ�2min����4minʱO2��ƽ����Ӧ����Ϊ mol��L��1��min��1
�۷�Ӧ�ڵ�2min�ı��˷�Ӧ�������ı������������ ������ĸ��ţ�
a��ʹ�ô��� b�������¶� c������ѹǿ d������O2��Ũ��
�ܷ�Ӧ�ڵ�8min�ı�ķ�Ӧ���������� ���ٴδﵽƽ��ʱ��NO��������� �����������С�����䡱��
��3�������£�����һ�δﵽƽ��ʱ�Ļ������ͨ��ˮ�У�Ȼ�������Һ�μ�b L��ˮ����Һ�����ԣ���μӰ�ˮ�Ĺ����У���Һ��ˮ�ĵ���ƽ�⽫ (����� ������)�ƶ������μӰ�ˮ��Ũ��Ϊ mol��L��1��(NH3��H2O�ĵ���ƽ�ⳣ��Kb��2��10��5 mol��L��1)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֽ�0.2molCH3COONa����ͱ����2.24��HC1����ͬʱ�ܽ���ͬһ�ձ���ˮ�У��Ƶ�1L��Һ��������Һ��c��CH3COO�D��>c��C1�D�����������ж��в���ȷ���ǣ� ��
A��pH��7��298K��
B��c��CH3COOH��+c��CH3COO�D��=0.20mol?L�D1
C��c��CH3COOH��<c��CH3COO�D��
D��c��CH3COO�D��+c��OH�D��=0.10mol?L�D1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08ɽ��ʡ��ˮ������ϣ��ֽ�0.2molCH3COONa����ͱ����2.24��HCl����ͬʱ�ܽ���ͬһ�ձ���ˮ�У��Ƶ�1L��Һ��������Һ��c��CH3COO�D��>c��Cl�D�����������ж��в���ȷ���ǣ� ��
A��pH��7��298K��
B��c��CH3COOH��+c��CH3COO�D��=0.20mol?L�D1
C��c(CH3COOH)<c(CH3COO�D)
D��c��CH3COO�D��+c��OH�D��=0.10mol?L�D1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com