
���� ��1��ͨ����ʯ�Ҽ�С��11.2L����ΪCO2��n��CO2��=$\frac{11.2L}{22.4L/mol}$=0.5mol���л���Xȼ�պ�ʣ�����������Ϊ��V��O2��=17.76 L-11.2 L=6.56 L��11.8g���л�����ȫȼ���������������ʵ���Ϊ��$\frac{20L-6.56L}{22.4L/mol}$=0.6mol��
11.8g�л���ȼ�ղ�����0.5 mol CO2�����л����к���0.5molCԪ�أ�0.5molC��ȫȼ������0.5molO2��ʣ���0.1molO2��0.4molHԪ�����ģ����л������дΪC0.5H0.4��H2O��x��18g/mol��x mol=11.8g-12g/mol��0.5mol-1g/mol��0.4mol���ݴ˼����x��Ȼ������Է�������С��120ȷ�������ʽ��
��2����Ũ���������£�1mol�л���Xʧȥ1molˮ�����л���Y��YΪ��״�ṹ����֧�����÷�ӦΪ������Ӧ��˵��X�����к����Ȼ����ǻ�������֧�����ݴ�ȷ��X�Ľṹ��ʽ��
��3�����������Ӧԭ����X�Ľṹ��ʽȷ��Y�Ľṹ��ʽ��
��� �⣺��1��ͨ����ʯ�Һ����������С��11.2LΪCO2��n��CO2��=$\frac{11.2L}{22.4L/mol}$=0.5mol���л���Xȼ�պ�ʣ�����������Ϊ��V��O2��=17.76 L-11.2 L=6.56 L���������������ʵ���Ϊ��$\frac{20L-6.56L}{22.4L/mol}$=0.6mol��
11.8g�л���ȼ�ղ�����0.5 mol CO2�����л����к���0.5molCԪ�أ�0.5molC��ȫȼ������0.5molO2��ʣ���0.1molO2��0.4molHԪ�����ģ����л������дΪC0.5H0.4��H2O��x��18g/mol��x mol=11.8g-12g/mol��0.5mol-1g/mol��0.4mol����ã�x=0.3mol��
���л�����Ա�ʾΪ��C0.5H0.4��H2O��0.3�������÷���ʽ�ɵã�C5H10O3������Է�������Ϊ118��120���������⣬
��X����Է�������Ϊ118��
��2����Ũ���������£�1mol�л���Xʧȥ1molˮ�����л���Y��YΪ��״�ṹ����֧����˵�����л�����Ũ������������ˮ�γɻ�״��������Ӧ�����Ȼ��������ǻ�����ṹ��ʽΪ��HOCH2CH2CH2CH2COOH��
�𣺸��л���Ľṹ��ʽΪHOCH2CH2CH2CH2COOH��
��3����Ũ���������£�1mol�л���HOCH2CH2CH2CH2COOHʧȥ1molˮ�����л���Y��Y�Ľṹ��ʽΪ��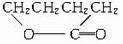 ��
��
��Y�Ľṹ��ʽΪ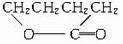 ��
��
���� ���⿼���л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷȷ��X�ķ���ʽΪ���ؼ���ע�����ճ����л�����ɡ��ṹ�����ʣ�����������ѧ���ķ�����������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ƿ�ͷ�Һ©��ʹ��ǰ����Ҫ��©������ | |
| B�� | �����þƾ���ȡ�ܽ���ˮ�еĵ� | |
| C�� | ������ˮӦ��������ɫϸ��ƿ�� | |
| D�� | ij��Һ��ɫ��Ӧ�ʻ�ɫ��˵����Һ�к���Na+������K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ��� | a | b |
| �Լ� | 0.1mol/L Fe��NO3��3 | 0.05mol/L Fe2��SO4��3 |
| ���� | ������ʧ�Ͽ죻 ��Һ��ɫ�Ա�dz�� ����������ɫ���� | ������ʧ������ ��Һ��ɫ���Ա�dz�� �����϶��ɫ���� |
| ʵ�鷽�� | Ԥ������ͽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
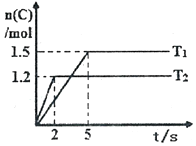 �ֽ�2molA��1molB����2L�ܱ������з�����Ӧ��2A��g��+B��g��?2C��g�����ֱ���Tl��T2ʱ���������C�����ʵ�����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������
�ֽ�2molA��1molB����2L�ܱ������з�����Ӧ��2A��g��+B��g��?2C��g�����ֱ���Tl��T2ʱ���������C�����ʵ�����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | T1��T2 | |
| B�� | �÷�Ӧ���¶�ΪT1ʱ�ﵽƽ��ʱ��������A��C�����ʵ���Ũ����� | |
| C�� | �¶�ΪT2ʱ��2s��B��ƽ������Ϊ0.3mol•L-1•s-l | |
| D�� | �¶�ΪT1ʱ����ƽ��ʱ��Ӧ��A��ת����Ϊ60% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
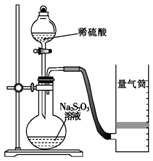 ������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮
������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮| ʵ����� | ���V/mL | ʱ��/s | |||
| Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
| �� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
| �� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
| �� | 6.0 | 2.0 | 4.0 | Vx | t3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com