
Įņ“śĮņĖįÄĘ(Na2S2O3)æÉÓĆ×ö·ÖĪöŹŌ¼Į¼°÷·øļµÄ»¹Ō¼Į£¬ĖüŹÜČČ”¢ÓöĖįŅ×·Ö½ā”£¹¤ŅµÉĻæÉÓĆ·“Ó¦£ŗ2Na2S£«Na2CO3£«4SO2===3Na2S2O3£«CO2 ÖʵƔ£ŹµŃéŹŅÄ£ÄāøĆ¹¤Ņµ¹ż³ĢµÄ×°ÖĆČēĶ¼ĖłŹ¾”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)bÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_______________________________________________________£¬
cÖŠŹŌ¼ĮĪŖ____________”£
(2)·“Ó¦æŖŹ¼ŗó£¬cÖŠĻČÓŠ»ė×Ē²śÉś£¬ŗóÓÖ±ä³ĪĒ唣“Ė»ė×ĒĪļŹĒ____________”£
(3)dÖŠµÄŹŌ¼ĮĪŖ______________”£
(4)ŹµŃéÖŠŅŖæŲÖĘSO2Éś³ÉĖŁĀŹ£¬æÉŅŌ²ÉČ”µÄ“ėŹ©ÓŠ
________________________________________________________________________
__________________________________________________________(Š“³öĮ½Ģõ)”£
(5)ĪŖĮĖ±£Ö¤Įņ“śĮņĖįÄĘµÄ²śĮ棬ŹµŃéÖŠĶØČėSO2²»ÄܹżĮ棬ŌŅņŹĒ________________________________________________________________________
________________________________________________________________________ӣ
“š°ø””(1)SO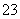 £«2H£«===SO2”ü£«H2O»ņHSO
£«2H£«===SO2”ü£«H2O»ņHSO £«H£«===SO2”ü£«H2O””Įņ»ÆÄĘŗĶĢ¼ĖįÄĘ»ģŗĻČÜŅŗ
£«H£«===SO2”ü£«H2O””Įņ»ÆÄĘŗĶĢ¼ĖįÄĘ»ģŗĻČÜŅŗ
(2)Įņ
(3)NaOHČÜŅŗ
(4)æŲÖĘ·“Ó¦ĪĀ¶Č”¢µ÷½ŚĖįµÄµĪ¼ÓĖŁ¶Č(»ņµ÷½ŚĖįµÄÅØ¶ČµČ)
(5)ČōSO2¹żĮ棬ČÜŅŗĻŌĖįŠŌ£¬²śĪļ·Ö½ā
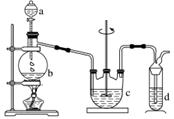
½āĪö””øł¾Ż×°ÖĆĶ¼æÉÖŖ£¬×ī×ó²ą×°ÖĆŹĒÖʱøSO2£¬ÖŠ¼ä×°ÖĆÓĆĄ“ÖʱøĮņ“śĮņĖįÄĘ(Na2S2O3)£¬ÓŅ²ą×°ÖĆŹĒĪ²Ęų“¦Ąķ×°ÖĆ(ĪüŹÕSO2)”£
(1)bÓĆĄ“ÖʱøSO2£¬ŹµŃéŹŅ³£ÓĆŃĒĮņĖįÄĘ(»ņŃĒĮņĖįĒāÄĘ)ŗĶĮņĖį·“Ӧɜ³É¶žŃõ»ÆĮņ”¢ĮņĖįÄĘŗĶĖ®£¬Ąė×Ó·½³ĢŹ½ĪŖSO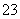 £«2H£«===SO2”ü£«H2O»ņHSO
£«2H£«===SO2”ü£«H2O»ņHSO £«H£«===SO2”ü£«H2O£»øł¾ŻÖĘČ”Įņ“śĮņĖįÄĘ(Na2S2O3)µÄ·½³ĢŹ½2Na2S£«Na2CO3£«4SO2===3Na2S2O3£«CO2£¬æÉÖŖcÖŠŹŌ¼ĮĪŖĮņ»ÆÄĘŗĶĢ¼ĖįÄĘ»ģŗĻČÜŅŗ”£
£«H£«===SO2”ü£«H2O£»øł¾ŻÖĘČ”Įņ“śĮņĖįÄĘ(Na2S2O3)µÄ·½³ĢŹ½2Na2S£«Na2CO3£«4SO2===3Na2S2O3£«CO2£¬æÉÖŖcÖŠŹŌ¼ĮĪŖĮņ»ÆÄĘŗĶĢ¼ĖįÄĘ»ģŗĻČÜŅŗ”£
(2)ŅņĪŖSO2¾ßÓŠŃõ»ÆŠŌ£¬ČÜŅŗÖŠ“ęŌŚS2££¬ĖłŅŌ¶žÕßÄÜ·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³Éµ„ÖŹS”£
(3)dŹĒĪ²Ęų“¦Ąķ×°ÖĆ(ĪüŹÕSO2)£¬ĖłŅŌdÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒNaOHČÜŅŗ”£
(4)æŲÖĘSO2Éś³ÉĖŁĀŹ£¬æÉŅŌ²ÉČ”æŲÖĘ·“Ó¦ĪĀ¶Č”¢µ÷½ŚĖįµÄµĪ¼ÓĖŁ¶Č(»ņµ÷½ŚĖįµÄÅضČ)µÄ·½·Ø”£
(5)Įņ“śĮņĖįÄĘ(Na2S2O3)ŹōÓŚĒæ¼īČõĖįŃĪ£¬ÓėĖįČŻŅ×·¢Éś·“Ó¦(S2O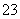 £«2H£«===S”ż£«SO2”ü£«H2O)£¬ČōSO2¹żĮ棬ŌņČÜŅŗĻŌĖįŠŌ£¬Įņ“śĮņĖįÄĘ(Na2S2O3)¾Ķ·¢Éś·“Ó¦µ¼ÖĀ²śĘ·ÖŹĮæ¼õÉŁ”£
£«2H£«===S”ż£«SO2”ü£«H2O)£¬ČōSO2¹żĮ棬ŌņČÜŅŗĻŌĖįŠŌ£¬Įņ“śĮņĖįÄĘ(Na2S2O3)¾Ķ·¢Éś·“Ó¦µ¼ÖĀ²śĘ·ÖŹĮæ¼õÉŁ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŌ×ÓµÄ3dÄܼ¶ÖŠÓŠŅ»øöµē×Ó£¬Ę䵌ĖIJćÖŠµÄµē×ÓŹżĪŖ(””””)
A£®0 B£®2 C£®3 D£®8
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹż¾ŻŹĒ¶ŌÓ¦ĪļÖŹµÄČŪµć£ŗ
| Na2O | NaCl | AlF3 | AlCl3 |
| 920 | 801 | 1 291 | 190 |
| BCl3 | Al2O3 | CO2 | SiO2 |
| £107 | 2 073 | £57 | 1 723 |
¾Ż“Ė×÷³öµÄĻĀĮŠÅŠ¶ĻÖŠ“ķĪóµÄŹĒ(””””)
A£®ĀĮµÄ»ÆŗĻĪļµÄ¾§ĢåÖŠÓŠµÄŹĒĄė×Ó¾§Ģå B£®±ķÖŠÖ»ÓŠBCl3ŗĶøɱłŹĒ·Ö×Ó¾§Ģå
C£®Ķ¬×åŌŖĖŲµÄŃõ»ÆĪļæÉŠĪ³É²»Ķ¬ĄąŠĶµÄ¾§Ģå D£®²»Ķ¬×åŌŖĖŲµÄŃõ»ÆĪļæÉŠĪ³ÉĻąĶ¬ĄąŠĶµÄ¾§Ģå
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ŹĒ¼ģŃéijĪŽÉ«ĘųĢåAŹĒSO2ŗĶCO2µÄ»ģŗĻĘųĢåµÄ×°ÖĆĶ¼£¬°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā”£
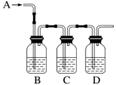
(1)BÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ________£¬×÷ÓĆŹĒ______________________________________________”£
(2)CÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ__________£¬×÷ÓĆŹĒ__________________________________________”£
(3)DÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ________£¬×÷ÓĆŹĒ___________________________________________”£
(4)ŹµŃ鏱£¬CÖŠÓ¦¹Ū²ģµ½µÄĻÖĻóŹĒ______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆĻĀĆęµÄ·½°ø½ųŠŠÄ³Š©Ąė×ӵļģŃ飬ĘäÖŠ·½°øÉč¼Ę×īŃĻĆܵďĒ(””””)
A£®¼ģŃéŹŌŅŗÖŠµÄSO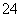 £ŗŹŌŅŗ
£ŗŹŌŅŗ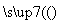 ĪŽ³Įµķ
ĪŽ³Įµķ °×É«³Įµķ
°×É«³Įµķ
B£®¼ģŃéŹŌŅŗÖŠµÄSO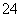 £ŗŹŌŅŗ
£ŗŹŌŅŗ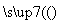 ĪŽ³Įµķ
ĪŽ³Įµķ °×É«³Įµķ
°×É«³Įµķ
C£®¼ģŃéŹŌŅŗÖŠµÄI££ŗŹŌŅŗ Éī»ĘÉ«ČÜŅŗ
Éī»ĘÉ«ČÜŅŗ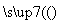 Éī»ĘÉ«ČÜŅŗ
Éī»ĘÉ«ČÜŅŗ
D£®¼ģŃéŹŌŅŗÖŠµÄCO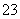 £ŗŹŌŅŗ
£ŗŹŌŅŗ °×É«³Įµķ
°×É«³Įµķ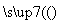 ³ĮµķČܽā
³ĮµķČܽā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ļ“µÓŗ¬SO2µÄŃĢĘų”£ŅŌĻĀĪļÖŹæÉ×÷Ļ“µÓ¼ĮµÄŹĒ________”£
a£®Ca(OH)2 b£®Na2CO3
c£®CaCl2 d£®NaHSO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲĮņŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄĖµ·Ø»ņĆčŹöÕżČ·µÄŹĒ(””””)
A£®Įņ»ĘæóÖʱøĮņĖį¾ĄśĮ½²½£ŗS SO3
SO3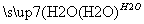 H2SO4
H2SO4
B£®ĖįÓźÓėĶĮČĄÖŠµÄ½šŹōŃõ»ÆĪļ·“Ó¦ŗó£¬ĮņŌŖĖŲŅŌµ„ÖŹµÄŠĪŹ½½ųČėĶĮČĄÖŠ
C£®ŌŚČ¼ĆŗÖŠ¼ÓČėŹÆ»ŅŹÆæɼõÉŁSO2ÅÅ·Å£¬·¢ÉśµÄ·“Ó¦ĪŖ2CaCO3£«2SO2£«O2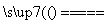 2CO2£«2CaSO4
2CO2£«2CaSO4
D£®ĶĮČĄÖŠµÄÉĮŠææó(ZnS)Óöµ½ĮņĖįĶČÜŅŗ×Ŗ»ÆĪŖĶĄ¶(CuS)£¬ĖµĆ÷CuSŗÜĪČ¶Ø£¬²»¾ßÓŠ»¹ŌŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°“ŅŖĒóĢīæÕ(¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ)
(1)×īĶā²ćµē×ÓŹżĪŖ1µÄŌŖĖŲÓŠ______(ĢīŌŖĖŲ·ūŗÅ£¬ĻĀĶ¬)”£
(2)×īĶā²ćµē×ÓŹżĪŖ2µÄŌŖĖŲÓŠ______”£
(3)×īĶā²ćµē×ÓŹżÓė“ĪĶā²ćµē×ÓŹżĻąµČµÄŌŖĖŲÓŠ______”£
(4)×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹż2±¶µÄŌŖĖŲŹĒ______”£
(5)×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹż3±¶µÄŌŖĖŲŹĒ______”£
(6)“ĪĶā²ćµē×ÓŹżŹĒ×īĶā²ćµē×ÓŹż2±¶µÄŌŖĖŲÓŠ______”£
(7)ÄŚ²ćµē×Ó×ÜŹżŹĒ×īĶā²ćµē×ÓŹż2±¶µÄŌŖĖŲÓŠ______”£
(8)µē×Ó²ćŹżÓė×īĶā²ćµē×ÓŹżĻąµČµÄŌŖĖŲÓŠ______”£
(9)×īĶā²ćµē×ÓŹżŹĒµē×Ó²ćŹż2±¶µÄŌŖĖŲÓŠ______”£
(10)×īĶā²ćµē×ÓŹżŹĒµē×Ó²ćŹż3±¶µÄŌŖĖŲÓŠ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ±ź×¼×“æöĻĀ½«1.92 gĶ·ŪĶ¶ČėŅ»¶ØĮæÅØHNO3ÖŠ£¬Ėę×ÅĶ·ŪµÄČܽā£¬·“Ӧɜ³ÉµÄĘųĢåŃÕÉ«Öš½„±äĒ³£¬µ±Ķ·ŪĶźČ«Čܽāŗó¹²ŹÕ¼Æµ½ÓÉNO2ŗĶNO×é³ÉµÄ»ģŗĻĘųĢå1.12 L£¬Ōņ·“Ó¦ĻūŗÄHNO3µÄĪļÖŹµÄĮæĪŖ(””””)
A£®0.8 mol B£®0.6 mol
C£®0.11 mol D£®ĪŽ·Ø¼ĘĖć
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com