��������һ�������£�2.30g����A��5.35gNH
4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C ����״����������C��������ˮ�õ�������Һ������֪CΪNH
3�������ˮB������һ�ֶ�����Ԫ�صĽ�������D��������BΪ����D�Ȼ��4.48L���������ʵ���=
=0.2mol��������=0.2mol��17g/mol=3.4g�����������غ��֪B������Ϊ2.3g+5.35g-3.4g=4.25g��NH
4Cl��Ħ������Ϊ53.5g/mol��5.35gNH
4ClΪ0.1mol��A�к�Li����DΪ��A������������A��NH
4Cl���巴Ӧ�ɱ�Ϊ��A+NH
4Cl��LiCl+NH
3������Clԭ���غ㣬LiCl�����ʵ���=0.1mol����ô2.3g������A�к�LiԪ��ҲΪ 0.1mol���ٸ��������غ��ԭ���غ㣨ԭ�ӵ��������Ŀ��Ӧǰ����ͬ������2.3gA�к���Nԭ��Ϊ0.2mol-0.1mol=0.1mol������Hԭ��Ϊ0.2mol��4-0.4mol=0.2mol������֪A��LiNH
2�������ˮB�����ɽ�������D��������BΪLiCl�������DΪLi�����Ʊ����̿�֪����Ϊ����Ũ������ȴ�ᾧ���õ����壬����ʧȥˮ�õ���ˮLiCl���۵��õ�Li���Դ������
���
�⣺��һ�������£�2.30g����A��5.35gNH
4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C ����״����������C��������ˮ�õ�������Һ������֪CΪNH
3�������ˮB������һ�ֶ�����Ԫ�صĽ�������D��������BΪ����D�Ȼ��4.48L���������ʵ���=
=0.2mol��������=0.2mol��17g/mol=3.4g�����������غ��֪B������Ϊ2.3g+5.35g-3.4g=4.25g��NH
4Cl��Ħ������Ϊ53.5g/mol��5.35gNH
4ClΪ0.1mol��A�к�Li����DΪ��A������������A��NH
4Cl���巴Ӧ�ɱ�Ϊ��A+NH
4Cl��LiCl+NH
3������Clԭ���غ㣬LiCl�����ʵ���=0.1mol����ô2.3g������A�к�LiԪ��ҲΪ 0.1mol���ٸ��������غ��ԭ���غ㣨ԭ�ӵ��������Ŀ��Ӧǰ����ͬ������2.3gA�к���Nԭ��Ϊ0.2mol-0.1mol=0.1mol������Hԭ��Ϊ0.2mol��4-0.4mol=0.2mol������֪A��LiNH
2��
��1��������������֪��AΪLiNH
2��CΪ�����������ʽΪ
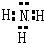
���ʴ�Ϊ��LiNH
2��
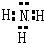
��
��2��������A�����ᷴӦ�Ļ�ѧ����ʽΪLiNH
2+2HCl=LiCl+NH
4Cl���ʴ�Ϊ��LiNH
2+2HCl=LiCl+NH
4Cl��
��3���٢�����A��750��800��ֽ�����E�Ͱ���������Ԫ���غ��֪��EΪ����ﮣ���ӦΪ3LiNH
2Li
3N+2NH
3���ʴ�Ϊ��3LiNH
2Li
3N+2NH
3��
�ھ��õ�����A���ñ���ױ����串�ǣ�Ȼ���������ñ���ױ�ϡ������ˮ�Ҵ�������LiNH
2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ����Ը�LiNH
2��Ӧ������ʽΪLiNH
2+C
2H
5OH=C
2H
5OLi+NH
3�ɽ������٣�
�ʴ�Ϊ��LiNH
2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ����Ը�LiNH
2��Ӧ������ʽΪLiNH
2+C
2H
5OH=C
2H
5OLi+NH
3�ɽ������٣�
��4���������̿�֪����Һ�еõ����壬������в�������Ϊ����Ũ������ȴ�ᾧ���ʴ�Ϊ������Ũ������ȴ�ᾧ��
����LiCl�qH
2O?LiCl+H
2O��֪��������м�ѹ��Ŀ���Ǽ�Сѹǿ������������ƽ�����������ƶ�����������ˮLiCl���Ʊ����ʴ�Ϊ����Сѹǿ��������LiCl�qH
2O?LiCl+H
2Oƽ�����������ƶ�����������ˮLiCl���Ʊ���


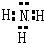 ���ʴ�Ϊ��LiNH2��
���ʴ�Ϊ��LiNH2��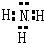 ��
��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
 ��2004��1��17�����ױ������������ѧ��ʹ����ͨ�����Ӻʹ�����������������ã��Ƴ������͵�������
��2004��1��17�����ױ������������ѧ��ʹ����ͨ�����Ӻʹ�����������������ã��Ƴ������͵�������