
�ϳɰ���ҵ�����õĦ��DFe��������Ҫ�ɷ���FeO��Fe2O3����֪��������Fe2+��Fe3+�����ʵ���֮��Ϊ1��2ʱ�����������ߡ�ij��ѧС����ͼ��Fe2O3Ϊԭ���Ʊ��ô���������֮һ�������м���̿�۸��·�Ӧ��2 Fe2O3 + C ![]() 4FeO+CO2����
4FeO+CO2����
ʵ���ʦ�ṩ��12g̿�ۡ�������㣬��Ҫ��ȡ���ֻ�����ߵĴ����������Fe2O3������Ϊ g��
��С������˶��ʵ�鷽�����ⶨ���ò�Ʒ�Ƿ����Ҫ������һ��ʵ�鷽������������ԭ���ȵĻ����ⶨ������������������п��ϡ���ᷴӦ��ȡ����ʱ����С���ͬѧ��ϡ�����м�����������ͭ��Һ��Ŀ���� �����������������������Ϊ ���û���ķ�����ʾ��ʱ���ò�Ʒ�ϸ�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 84(m1-m2) |
| 31m1 |
| 84(m1-m2) |
| 31m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
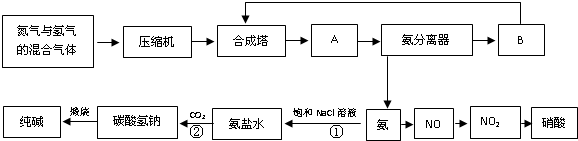
����6�֣����ʼҿ�ѧԺ��������2007��ŵ������ѧ������¹�����ѧ�ḥ���-������Fritz-Haber���о����ĸ���¡����ض�(Gerhard Ertl)���ڣ��Ա������ڹ�����滯ѧ�����о����������Ŀ����Գɾ͡��ϳɰ���ҵ���������õĦ�-Fe��������Ҫ�ɷ���FeO��Fe2O3��
��1��ijFeO��Fe2O3������У������������ʵ���֮��Ϊ4��5������Fe2+��Fe3+���ʵ���֮��Ϊ_________��
��2����������Fe2+��Fe3+���ʵ���֮��Ϊ1��2ʱ�����������ߣ���ʱ����������������������������Ϊ_________����С����ʾ����
��3����Fe2O3Ϊԭ���Ʊ������������������м�������̼�ۣ��������·�Ӧ��
2Fe2O3 +C��4FeO +CO2����Ϊ�Ƶ����ִ�������ߵĴ�����Ӧ��480g Fe2O3��ĩ�м���̼�۵�����Ϊ_________ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣������������ж�����;�����������й����⣺
��1�����������Ҫ�ɷ���Fe2O3���ǹ�ҵ��������Ҫԭ��֮һ��д����Fe2O3�Ƶõ������Ļ�ѧ����ʽ ��ij��������˺�Fe2O3֮����������������SiO2��Al2O3������������NaOH��Һ����ַ�Ӧ���ˣ����ɵõ��ϴ�����Fe2O3��������Ӧ�����ӷ���ʽ�� ��
��2��Fe2O3����������������ȼ����ں��Ӹֹ�����ơ�ijͬѧ�²⣬�÷�Ӧ�����������к���Fe2O3�����������ʵ������֤�Լ��IJ��룺ȡ������������������ ϡ���ᣬȻ��μ�KSCN��Һ��������Ѫ��ɫ��֤������Fe2O3����������Fe2O3��
����Ϊ�÷���������Ϊʲô�� ��
��3���ϳɰ���ҵ�����õĦ��DFe��������Ҫ�ɷ���FeO��Fe2O3����֪��������Fe2+��Fe3+�����ʵ���֮��Ϊ1��2ʱ�����������ߡ�ij��ѧС����ͼ��Fe2O3Ϊԭ���Ʊ��ô���������֮һ�������м���̿�۸��·�Ӧ��
![]()
ʵ���ʦ�ṩ��12g̿�ۡ�������㣬��Ҫ��ȡ���ֻ�����ߵĴ����������Fe2O3������Ϊ g�� ��С������˶��ʵ�鷽�����ⶨ���ò�Ʒ�Ƿ����Ҫ������һ��ʵ�鷽������������ԭ���ȵĻ����ⶨ������������������п��ϡ���ᷴӦ��ȡ����ʱ����С���ͬѧ��ϡ�����м�����������ͭ��Һ��Ŀ���� �����������������������Ϊ ���û���ķ�����ʾ��ʱ���ò�Ʒ�ϸ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com