
| Ӧ����NaOH������/g | �Ѹ����� | ���Ѹ��������Ҫ���������� |
| | �ձ���ҩ�ס� ������ƽ | |
| A������NaOH����ʱ��¶���ڿ�����ʱ����� |
| B��ѡ�õ�����ƿ��������������ˮ |
| C�����ձ����ܽ�NaOH��������������Һע������ƿ�� |
| D���ڶ���ʱ��������ƿ�̶��� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ͨ��ˮ���ܵ�NH3��H2O | B�����Ȼ�̼��ȡ��ˮ�еĵ� |
| C�����˳�ȥ�����еIJ��������� | D��������ˮ����Ϊ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A�����ֶ�ס����©�����ڵ���ĩ�˽�һ����Ƥ����������ס���ƿ������Ƥ������������ж�װ�ò�©�� |
| B�����ƿ�ڼ������ʯ��ϡ���ᣬ����©���¶˹ܿڽ���Һ�����£��ձ���ע��ˮ�����ձ��г������ݣ����ж�װ�ò�©�� |
| C���ӳ���©�������´��������ձ����Ƿ�������ݣ����ж�װ���Ƿ�©�� |
| D���ֱ�����ƿ���ձ���ע������ˮ��ʹ����©���¶˺͵��ܿڶ�����ˮ�����£�Ȼ��������ס���ƿ��������ܿ�������ð���������ƿ�����һ���ձ��е�ˮ������������γ�һ��ˮ�������ж�װ�ò�©�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Թ���Һ����ȣ�Ӧ�Ⱦ������ȣ�Ȼ��С�ĵ����Թ�Һ������²����� |
| B����������������������壬���ܽ���ˮ���峴�� |
| C����ֽʹ��ʱ�ȼ���С�鲢���ڱ��������Ƭ�ϣ�Ȼ���øɾ��IJ�����պȡ��Һ������ֽ���в���������Һ������ |
| D��ȡ����ˮ��Һ��NaOH�к������ԣ��������Ƶ�Cu��OH��2����Һˮԡ���ȣ���ɫ������֤������δˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Һ�IJ����У�ת����Һ���������ձ�δϴ�ӻ�ʹ��������ҺŨ��ƫ�� |
| B��ϴ�ӳ����IJ����ǽ��������ڹ������У����ò��������������ˮ��ϴ |
| C��ʵ��������950 mL 0.2 mol/L��CuSO4��Һʱ�����ȡ����������Ϊ50.0 g |
| D���Ʊ�Fe(OH)3����ʱ�������͵�FeCl3��Һ�����ˮ�У����ȱ߽��裬ֱ���õ����ɫ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
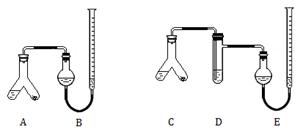
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ɹ��˽�״���� | B���ɹ��˿�����С�ij��� |
| C����ʹ�����е����ʼ��� | D���ɵõ��ϸ���ij��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Ը��������Һ�����顢�����ױ� |
| B����ˮ���𱽺��屽 |
| C����ȡ�ܽ���ˮ�е������⣺�����ѻ������� |
| D����ȡ�屽������м��Һ�塢����ֻ�Ϻ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com