
����˵����ȷ����
A����֪2SO2��g��+ O2��g�� 2SO3 ��g�� ��H��0���÷�Ӧ���κ��¶��¾����Է�����
2SO3 ��g�� ��H��0���÷�Ӧ���κ��¶��¾����Է�����
B��0.01 mol��L-1NaHCO3��Һ��c��Na����= c��HCO3 -��+ 2c��CO32-��+ c��H2CO3��
C��25�棬0.1mol��L-1 K2CO3��Һ��c��H+��/ c��OH���� =l.0 �� l0-a������Һ��pH=7+0.5a
D�������£�Ksp��AgCl��=1.8��10-10��Ksp��Ag2CrO4��=9.0��10-12����Ũ����ȵ�Na2CrO4��NaCl�Ļ��ϡ��Һ�еμ�0.01 mol��L-1 AgNO3��Һ��������Ag2CrO4����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ŷŵ�β���к���NO2��NO2�dz��д�����Ⱦ����Ҫ��Ⱦ��֮һ�����չ������£�NO2����һϵ�й⻯ѧ������ѭ����Ӧ���Ӷ����ϲ���O3�����ؿ�����Ⱦ����Ӧ����Ϊ��2NO2����2NO��2O����2NO��O2����2NO2����O��O2����O3�����жԸ÷�Ӧ���̼��������� ����ȷ���� (����)
����ȷ���� (����)
A��NO2��������� B��NO���������
C��NO2ֻ������������ D��O3��O2Ϊͬ���칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ����С����ʵ�����������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣
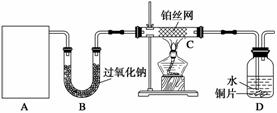
(1)A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������__________(��ѡ����)����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ____________(ѡ������������ţ��̶�װ��ʡ��)��
(2)��װ�ò�����������Ȼ����һ����ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
��________________________________________________________________________��
��________________________________________________________________________��
(3)���ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B��������_____________________________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ�����������һ��������ͭ�ۣ�������ܽ�19.2 g(��֪����ֻ����ԭΪNO����)������һ�����������ۣ�������������������������ӵı仯������ͼ��ʾ��

������������(����)
A��AB�εķ�ӦΪ��Fe��2Fe3��===3Fe2��
B���ڶ�����Һ����������ΪFeSO4
C���������NO ���ʵ���Ϊ0.4 mol
���ʵ���Ϊ0.4 mol
D���������H2SO4Ũ��Ϊ5 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���Ľṹ��ʽ����ͼ��ʾ�����й��ڸ����ʵ�����������ȷ����
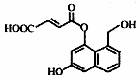 A������ʹ������Ȼ�̼��Һ��ɫ
A������ʹ������Ȼ�̼��Һ��ɫ
B���ܷ������۷�Ӧ
C�����ܷ�����ȥ��Ӧ��Ҳ�ܷ���ȡ����Ӧ
D��1 mol������������5mol NaOH��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ��ѡ��3���ʽṹ������]��15�֣�
X��Y��Z��W��R��TΪǰ������Ԫ����ԭ��������������Tԭ����������X��Y��Rԭ������֮�͡�ZΪ�ؿ��к�������Ԫ�ء�X��Zԭ�Ӻ������2��δ�ɶԵ��ӡ�Z��Rλ��ͬһ���塣X��Y��Z��W��R��T��ֻ�����ֽ���Ԫ�أ��Ҵ������з�Ӧ��
2W+XZ2  X+2WZ
X+2WZ
�ش��������⣺
��1��X��Y��Z�ĵ�һ������������ ����Ԫ�ط��ű�ʾ����
��2����Ԫ��R��Ԫ��Z�γɵij����������У����ڷǼ��Է��ӵ��� ���ѧʽ�����÷���������ԭ���� �ӻ���
��3����X��TԪ����ɵĵ�����һ�������� ������ţ���
A�����Ӿ��� B�����Ӿ��� C��ԭ�Ӿ��� D����������
��4����̬Tԭ�ӵĺ�������Ų�ʽΪ ��
��5��T+����NH3ͨ����λ�����Ϊ[T(NH3)n]+����������T+��4s�����4p���ͨ��sp�ӻ�����NH3�ṩ�ŵ��Ӷԡ�
�� [T(NH3)n]+��n= ��
�� [T(NH3)n]+��T+��n����ԭ�ӹ��ɵĿռ�ṹ�� �͡�
 ��6��������WZ��NaCl�ľ����ṹ����
��6��������WZ��NaCl�ľ����ṹ����
���Ȼ��ƾ����ṹ����ͼ��ʾ����
����WZ�У������Ӻ�����
�ӵ���λ����Ϊ ��
����֪WZ���ܶ�Ϊa g/cm3��
��WZ�о�������������Ӽ�ľ���Ϊ
pm���ú�a����ʽ��ʾ������٤������ΪNA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й����ʴ�����ͼ��ʾ��ת����ϵ(���ֲ�������ȥ)��ͨ��CΪ���嵥�ʣ�GΪ�Ϻ�ɫ���嵥�ʡ�ʵ�����У����ù���E��B�Ĵ��¼�����ȡ���嵥��H��

��ش��������⣺
(1)��Ӧ�ٵĻ�ѧ����ʽΪ_________________________________________________
________________________________________________________________________��
(2)��Ӧ�ڵ����ӷ���ʽΪ__________________________________________________
[
________________________________________________________________________��
(3)д������һ��ʵ������ȡH�Ļ�ѧ����ʽ��_______________________________
________________________________________________________________________��
(4)D��Һ��Pb(NO3)2��Һ��Ͽ��γɳ������˳�����Ksp��7.0��10��9�����������D��Һ��Pb(NO3)2��Һ��ϣ���D��Ũ��Ϊ1��10��2 m ol·L��1�������ɳ�������Pb(NO3)2��Һ����
ol·L��1�������ɳ�������Pb(NO3)2��Һ���� СŨ��Ϊ______________��
СŨ��Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����Ħ������ΪM g·mol��1��NA��ʾ�����ӵ�������ֵ����һ�����¶Ⱥ�ѹǿ�£����ΪV L�ĸ����������еķ�����ΪX���� ��ʾ���� (����)
��ʾ���� (����)
A��V L�����������(��gΪ��λ)
B��1 L�����������(��gΪ��λ)
C��1 mol����������(��LΪ��λ)
D��1 L�������������ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)Ϊ�˷�ֹˮԴ��Ⱦ���ü����������Եķ��� ����ij�����ŷŵ���ˮ�к��б��ӣ�ʵ�������___________________________________________________���ӷ�ˮ�л��ձ��ӵķ����ǣ���ȡ�л��ܼ���ȡ��Һ�еı��ӣ��ڼ���ij��ҩƷ��ˮ��Һʹ���Ӵ��л��ܼ������룻��ͨ��ij�������������ӡ���д���ڡ��۲��Ļ�ѧ����ʽ______________________________;__________________________________��
��2��ijȩ�Ľṹ��ʽΪ 
���������ȩ�������õ��Լ���________________����ѧ����ʽΪ____________;
Ȼ������Ӧ�����Һ�м���ϡ��������Һ�����ԣ��ٵμ���������ˮ�����������̼̼˫�������ɵ��л�����Ľṹ��ʽ��____________;
(3)����������Ӧ���Թ��ڱڵ�һ����������_________��ȥ����д��ѧʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com