
��10�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ���������� ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��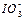 ��Na+��Ũ�ȹ�ϵ�� ������ڡ���С�ڡ����ڡ�����
��Na+��Ũ�ȹ�ϵ�� ������ڡ���С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�����ʡ����ʦ���и����������¿���ѧ�Ծ� ���ͣ������
��8�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��IO��3��Na+�����ʵ����Ĺ�ϵ��n(Na+) n(IO-3)������ڡ�����С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����������¿���ѧ�Ծ� ���ͣ������
��8�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��IO��3��Na+�����ʵ����Ĺ�ϵ��n(Na+) n(IO-3)������ڡ�����С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
��10�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ���������� ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��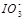 ��Na+��Ũ�ȹ�ϵ��
������ڡ���С�ڡ����ڡ�����
��Na+��Ũ�ȹ�ϵ��
������ڡ���С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��IO��3��Na+�����ʵ����Ĺ�ϵ��n(Na+) n(IO-3)������ڡ�����С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com