
¢ń.ČēĶ¼ĖłŹ¾£¬ŌŚ“óŹŌ¹ÜĄļ·ÅČėŅ»¶Ī¹āĮĮĪŽŠāµÄĶä³ÉĀŻŠż×“
µÄĢśĖ棬°ŃŹŌ¹Üµ¹²åČėĖ®ÖŠ£¬°ŃÕāøö×°ÖĆÕāŃł·ÅÖĆŌ¼Ņ»ÖÜŗ󣬹Ū²ģ
µ½ĢśĖæ·¢ÉśµÄ±ä»ÆŹĒ______________________£¬ŌŅņŹĒ
____________________£®ŹŌ¹ÜĄļµÄĖ®Ćę»įÉĻÉż£¬×īÖÕÉĻÉżø߶ČŌ¼ĪŖ
________£¬ŌŅņŹĒ_________________________________________
________________________________________________________________________.
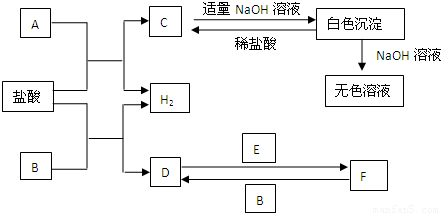
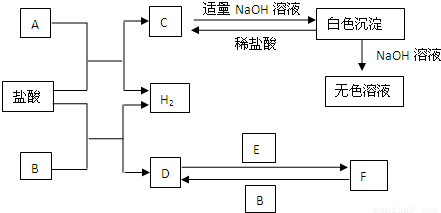
¢ņ.A”¢B”¢CČżøöÉÕ±ÖŠ·Ö±šŹ¢ÓŠĻąĶ¬ĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖį£®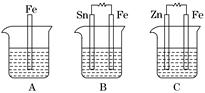
(1)AÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________________£®
(2)BÖŠSnµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ________________________________________£¬
Snµē¼«ø½½üČÜŅŗµÄpH__________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)£®
(3)CÖŠ±»øÆŹ“µÄ½šŹōŹĒ________£¬×Ü·“Ó¦Ź½ĪŖ________________________£®±Č½Ļ
A”¢B”¢CÖŠĢś±»øÆŹ“µÄĖŁĀŹ£¬ÓÉæģµ½ĀżµÄĖ³ŠņŹĒ______________________£®
¢ń.ĢśĖæ±ķĆęÉś³ÉŅ»²ćŗģ×ŲÉ«µÄĢśŠā””Ģś·¢ÉśĪüŃõøÆŹ“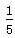 æÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹżŌ¼ĪŖ1/5£¬¾¹ż×ć¹»³¤µÄŹ±¼äŗó£¬ŃõĘųÓėĢś·“Ó¦Ķź
æÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹżŌ¼ĪŖ1/5£¬¾¹ż×ć¹»³¤µÄŹ±¼äŗó£¬ŃõĘųÓėĢś·“Ó¦Ķź
¢ņ.(1)Fe£«2H£«===Fe2£«£«H2”ü
(2)2H£«£«2e£===H2”ü””Ōö“ó
(3)Zn””Zn£«2H£«===Zn2£«£«H2”ü””B£¾A£¾C
½āĪöŹŌĢā·ÖĪö£ŗøĆ×°ÖĆÖŠĢśĖæ·¢ÉśĪüŃõøÆŹ“Éś³ÉĢśŠā(Fe2O3”¤nH2O)£¬¾¹ż×ć¹»³¤µÄŹ±¼äŗó£¬ŹŌ¹ÜÄŚæÕĘųÖŠµÄŃõĘųĶźČ«±»ĪüŹÕ£¬µ¼ÖĀŃ¹Ēæ¼õŠ”£¬ŅŗĆęÉĻÉż£¬ÉĻÉżø߶ČÓėæÕĘųÖŠĖłŗ¬ŃõĘųµÄĢå»ż·ÖŹżĻąĶ¬£¬Ō¼Õ¼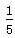 .
.
(¢ņ)AÖŠFeŹ§µē×Ó£¬ĒāĄė×ӵƵ½µē×Ó±»»¹ŌĪŖĒāĘų£»BÖŠŠĪ³ÉŌµē³Ų£¬SnĪŖÕż¼«£¬ĪŖµē×ÓĮ÷ČėµÄŅ»¼«£¬ĒāĄė×ӵƵē×Ó±»»¹Ō²śÉśĒāĘų£¬ÓÉÓŚĻūŗÄĮĖČÜŅŗÖŠµÄĒāĄė×Ó£¬ĖłŅŌpHŌö“ó£»CÖŠŠæ×÷øŗ¼«±»Ńõ»Æ£¬Zn£2e£===Zn2£«£¬Õż¼«£ŗ2H£«£«2e£===H2”ü£¬×Ü·“Ó¦ĪŖZn£«2H£«===Zn2£«£«H2”ü£¬CÖŠFe±»±£»¤£¬BÖŠÄܼÓæģøÆŹ“£®
æ¼µć£ŗŌµē³Ų¼°µē½ā³Ų
µćĘĄ£ŗ±¾Ģāæ¼²éĢśµÄøÆŹ“Ļą¹ŲµÄµē»ÆѧÖŖŹ¶£¬ÄŃ¶Č²»“󣬲ąÖŲæ¼²éѧɜŹµŃé¹Ū²ģÄÜĮ¦¼°·ÖĪöĪŹĢāÄÜĮ¦£¬½āĢāµÄ¹Ų¼üŹĒŅŖ¶Į¶®Ķ¼£¬ĮĖ½āĪŹĢāµÄŹµÖŹ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğĢģ½ņŹŠŗĶĘ½ĒųøßŅ»£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½Ī÷Ź”¼Ŗ°²ŹŠ¼ŖÖŻĒų°×šŲ֎֊ѧø߶ž£ØÉĻ£©ĘŚÖŠ»ÆѧŹŌ¾ķ£ØĪÄæĘ£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com