
| A�� | ϡ��ǰ����ҺpH���٣���=�� | |
| B�� | ϡ�ͺ���ҺpH���٣���=�� | |
| C�� | ϡ��ǰ����Һ�����ʵ����ʵ���Ũ�ȣ��ۣ���=�� | |
| D�� | ϡ�ͺ���Һ�����ʵ����ʵ���Ũ�ȣ��ۣ���=�� |
���� A��������ǿ�ᣬ����ȫ���룬���������ᣬ������ȫ���룻
B����ϡ�ͺ���Һ�е�������Ũ�Ȼ��С����������ĵ�����ܵ��ٽ���
C��pH=1�Ĵ�����Һ��Ũ�ȴ���0.1mol/L��
D��ϡ������Һ����ٽ�����룮
��� �⣺A��������ǿ�ᣬ����ȫ���룬0.1mol/L���ᣬpH=1�����������ᣬ������ȫ���룬0.1mol/L���ᣬpH��1����pH���٣���=�ۣ���A��ȷ��
B����ϡ�ͺ���Һ�е�������Ũ�Ȼ��С��0.1mol/L������Һϡ�ͺ�pH=2��pH=1�Ĵ�����Һ��Ũ�ȴ���0.1mol/L������Һ��ϡ�ͺ�pH=1�Ĵ�����Һ�ĸ�С����pH˳���Ǣڣ��٣��ۣ���B����
C�����������ᣬ������ȫ���룬pH=1�Ĵ�����Һ��Ũ�ȴ���0.1mol/L������ϡ��ǰ����Һ�����ʵ����ʵ���Ũ�ȣ��ۣ���=�ڣ���C��ȷ��
D��ϡ�ʹ�������Һ����ٽ�����룬������Һ�����ʵ����ʵ���Ũ�ȣ��ۣ��ڣ��٣���D����
��ѡBD��
���� ���⿼��ѧ�����������ˮ��Һ�еĵ��룬��Ŀ�Ѷ��еȣ���ȷ������ʵĵ���ƽ�⼰��Ӱ��Ϊ���ؼ���ע�����ѧ֪ʶ��Ǩ�ƺ�Ӧ�ã�����������ѧ�������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | l mol H20�����γ������ĿΪ2NA | |
| B�� | ��״���£�11.2 L��ȩ�����ĦҼ���ĿΪ2NA | |
| C�� | �����£�46 g N02����������ĿΪNA | |
| D�� | pH=l������������S042-����ĿΪ0.05NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼ԭ����ʧȥ�����ĵ����γ����� | |
| B�� | ̼ԭ������������������ԭ�ӵ��������γɹ�ͬ���Ӷ� | |
| C�� | ����л����̼ԭ�ӿ����γɵ�����˫���������ȶ��ֳɼ���ʽ | |
| D�� | ����л������ԭ�ӡ���ԭ�ӡ�±��ԭ�ӷֱ��γ�2����3����1�����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ��Һ©�� | C�� | ����ƿ | D�� | ��ͷ�ι� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | Fe2+��Na+��Ba2+��Al3+ |
| ������ | Cl-��SO42-��NO3-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 125.0��CuSO4•5H2O ���� | B�� | 76.8����ˮCuSO4 ���� | ||
| C�� | 120.0��CuSO4•5H2O ���� | D�� | 40.0����ˮCuSO4���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ԭ�ӵĽṹʾ��ͼ�� | B�� | ���������ķ���ϩ�Ľṹ��ʽ��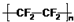 | ||
| C�� | ������Ϊ10�ķ�ԭ�ӣ�${\;}_{9}^{19}$F | D�� | өʯ��Ҫ�ɷ�CaF2����ʽ��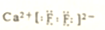 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ���Ƿ�����ԭ�Ӽ������� | |
| B�� | ���������ӵĻ�����һ������������ | |
| C�� | ���ӻ������۵�һ���ȹ��ۻ������ | |
| D�� | �ǽ���Ԫ����ɵ�һ���ǹ��ۻ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com