
��.����ͼ��ʾ����2molA�����1molB�������һ�ݻ��ɱ���ܱ������У�������Ӧ��2A��g��+B(g)  2C(g).��Ӧ��ʼʱ�ɻ����Ļ�����λ�����ͼ��ʾ������Ӧ�ﵽƽ��ʱ������λ������ͼ��ʾ���ﵽƽ��ʱ��A��ת����Ϊ
���������µķ�Ӧ��ƽ�ⳣ��Ϊ
��
2C(g).��Ӧ��ʼʱ�ɻ����Ļ�����λ�����ͼ��ʾ������Ӧ�ﵽƽ��ʱ������λ������ͼ��ʾ���ﵽƽ��ʱ��A��ת����Ϊ
���������µķ�Ӧ��ƽ�ⳣ��Ϊ
��
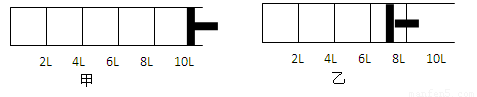
��.(1)��֪298Kʱ��1molC2H6����������ȫȼ�����ɶ�����̼��Һ̬ˮ���ų�����1558.3KJ��д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2�����ø÷�Ӧ�����һ��ȼ�ϵ�أ�������������Һ���������Һ���ö��ʯī���缫���ڵ缫�Ϸֱ���������������д�������ĵ缫��Ӧʽ ��
��3����ʯī���������������������������ͭ��Һ����ʯī���ϵĵ缫��ӦʽΪ �������ʼʱʢ��1000mL PH=5������ͭ��Һ��25�棩����������һ��ʱ�����Һ��PH��Ϊ1����Ҫʹ��Һ�ָ�����ʼŨ�ȣ�������Һ����仯����������Һ�м��� �����������ƣ���������ԼΪ ��
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
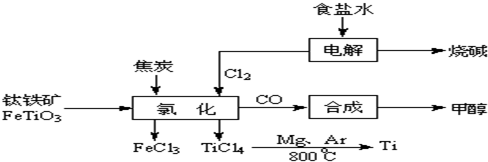
 2OH-+H2��+Cl2��
2OH-+H2��+Cl2�� 2OH-+H2��+Cl2��
2OH-+H2��+Cl2�� 2MgCl2��s��+Ti����Ar�����н��е������ǣ�
2MgCl2��s��+Ti����Ar�����н��е������ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
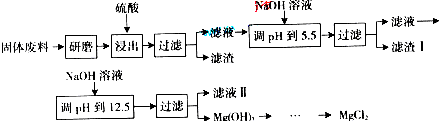
| ������ | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| PH | 3.2 | 5.2 | 12.4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com