
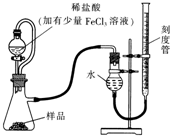
 ������þ��MgO2��������ϡ�ᣬ������������������⣬��ҽѧ�Ͽ���Ϊ����������ȣ�������þ��Ʒ�г������һ������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�����
������þ��MgO2��������ϡ�ᣬ������������������⣬��ҽѧ�Ͽ���Ϊ����������ȣ�������þ��Ʒ�г������һ������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ��������� ��1��ϡ�����м�������FeCl3��Һ��H2O2�ķֽ⣬������������������Ϊ�������������ӱ����������������������ӣ�
��2�����������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
��3��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
��4������b������þ������a�ǹ�����þ������þ��������������������ʵ�������ʽ������������ʵ����������ݹ�����þ�����ʵ������������þ������������
��5�����ڵ�����������ɫ����������������Һ��ָʾ����
��6�����ݵ����غ��ҳ���ϵʽ�������������þ������������
��7���ζ�ʵ����������ı���Һ����ⶨ���������Ҫ�ظ�2-3��ȡ�ⶨ��ƽ��ֵ��������
��� �⣺��1��ϡ�����м�������FeCl3��Һ��H2O2�ķֽ⣬������������������Ϊ��������Ӧ�����ӷ���ʽΪ��2Fe3++H2O2�T2Fe2++O2��+2H+���������ӱ����������������������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2�����������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
�ʴ�Ϊ������������Һ��������������������Ӱ�죻
��3����������������ѹǿӰ��������ڶ���֮ǰ��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
�ʴ�Ϊ�����Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��
��4���������þ�����ʵ�����xmol������þ���ʵ�����ymol����56x+40y=a����x+y����40=b�����x=$\frac{a-b}{16}$������I�й�����þ����������Ϊ��$\frac{\frac{a-b}{16}��56}{a}$��100%=$\frac{7��a-b��}{2a}$��100%��
�ʴ��ǣ�$\frac{7��a-b��}{2a}$��100%����
��5��������þ���������ԣ��ܰѵ⻯���������ɵ��ʵ⣬Ȼ��������������Ƶζ����ɵĵ��ʵ⼴�ɣ����ڵ�����������ɫ����������������Һ��ָʾ�����жϵ���ζ��յ����������ɫ����ɫ���μ����һ�α�Һ����Һ����ɫͻ����ɫ��������ڲ��ָ���
�ʴ�Ϊ��������Һ���μ����һ�α�Һ����Һ����ɫͻ����ɫ��������ڲ��ָ���
��6�����ݷ�Ӧ����ʽ��I2+2Na2S2O3=Na2S4O6+2NaI�����ݵ����غ��ҳ���ϵʽ��MgO2��I2������n��MgO2��=n��I2��=$\frac{1}{2}$n��Na2S2O3��=$\frac{1}{2}$��0.1mol/L��28.5��10-3L=1.425��10-3mol��
������þ�����������ǣ�$\frac{1.425��1{0}^{-3}mol��56g/mol}{0.1g}$��100%=79.8%=0.798��
�ʴ�Ϊ��0.798��
��7���ζ�ʵ����������ı���Һ����ⶨ���������Ҫ�ظ�2-3��ȡ�ⶨ��ƽ��ֵ����������ʵ��ȱ��ƽ��ʵ�飬
�ʴ�Ϊ����ʵ��ȱ��ƽ��ʵ�飮
���� ���⿼����ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ������������������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ��������������ջ����ǹؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2 | B�� | 1 | C�� | 3 | D�� | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ������Ľṹʽ�����У�������
������Ľṹʽ�����У�������| A�� | 1�� | B�� | 3�� | C�� | 2�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��ͼ��ʾA��B��C��D��E�������ʵ��ת����ϵ������AΪ����ɫ���壬CΪ�������ʣ�DΪ��õĵ�ζƷ��
��ͼ��ʾA��B��C��D��E�������ʵ��ת����ϵ������AΪ����ɫ���壬CΪ�������ʣ�DΪ��õĵ�ζƷ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

 ����Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ��
����Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵĺ˵�����������ڶ�����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������3���ܼ���Bԭ�ӵ������p����ĵ���Ϊ������ṹ��C�ǵؿ��к�������Ԫ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������ش��������⣺
A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵĺ˵�����������ڶ�����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������3���ܼ���Bԭ�ӵ������p����ĵ���Ϊ������ṹ��C�ǵؿ��к�������Ԫ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��Ƥ����ʹҺ��˳������ | B�� |  �����Ҵ������� | ||
| C�� |  ���װ�������� | D�� |  �ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com