
 Si3N4��s��+6CO��g��
Si3N4��s��+6CO��g��| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| N2Ũ��/mol��L-1 | 4��00 | 3��70 | 3��50 | 3��36 | 3��26 | 3��18 | 3��10 | 3��00 | 3��00 | 3��00 |
| COŨ��/mol��L-1 | 0��00 | 0��90 | 1��50 | 1��92 | 2��22 | 2��46 | 2��70 | | | |
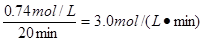 ��ƽ��ʱ������CO��Ũ�ȷֱ���3.00mol/L�ͣ�2.70��0.30��mol/L��3.00mol/L������ƽ�ⳣ��K��
��ƽ��ʱ������CO��Ũ�ȷֱ���3.00mol/L�ͣ�2.70��0.30��mol/L��3.00mol/L������ƽ�ⳣ��K��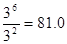 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��
2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________�� 4NH3(g)��3O2(g) ��H��Q��
4NH3(g)��3O2(g) ��H��Q�� �뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������
�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������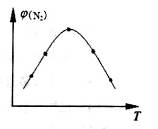
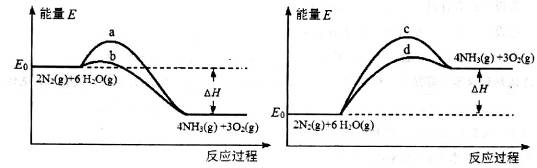
 2NH3(g) ��H����93.0kJ/mol��
2NH3(g) ��H����93.0kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������ L��
7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������ L�� 2SO3(g) ��H =" ��196.6" kJ��mol-1
2SO3(g) ��H =" ��196.6" kJ��mol-1 SO3(g)+NO(g) ��H = ��41.8kJ��mol-1
SO3(g)+NO(g) ��H = ��41.8kJ��mol-1 2NO2(g)�� ��H =" _________" kJ��mol-1
2NO2(g)�� ��H =" _________" kJ��mol-1 CH3OH��g������ƽ����ø����Ũ�����£�
CH3OH��g������ƽ����ø����Ũ�����£�| ���� | CO | H2 | CH3OH |
| Ũ��(mol?L��1) | 0.9 | 1.0 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 4NO2(g)��O2(g)����H��0
4NO2(g)��O2(g)����H��0| t/s | 0 | 500 | 1000 |
| c(N2O5)/mol��L��1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����¯�г�����CaSO4����Na2CO3��Һ���ݺ��ٽ���������ϡ�����ܽ�ȥ�� |
| B�����ˮ�еμ�FeCl3������Һ�Ʊ�Fe(OH)3�����ԭ���Ǽ��ȴٽ���Fe3+ˮ�� |
| C����ˮ�м���������¶���ʹˮ�����ӻ���С������ƽ�������ƶ� |
| D����Ӧ2A(g) + B(g) =" 3C" (s) + D(g)��һ�����������Է����У�˵���÷�Ӧ�Ħ�H>0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
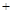 CO(g)
CO(g) CH3OH(g)
CH3OH(g)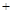 90.8 kJ
90.8 kJ CH3OCH3(g)
CH3OCH3(g) H2O(g)
H2O(g) 23.5 kJ
23.5 kJ H2O(g)
H2O(g) CO2(g)
CO2(g) H2(g)
H2(g) 41.3 kJ
41.3 kJ 3CO(g)
3CO(g) CH3OCH3(g)
CH3OCH3(g) CO2(g)
CO2(g)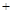 Q������Q
Q������Q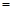 ����������kJ��
����������kJ�� CH3OCH3(g)
CH3OCH3(g)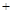 H2O(g)��һ�ܱ������н��е�10minʱǡ�ô�ƽ�⣬��ø���ֵ�Ũ�����£�
H2O(g)��һ�ܱ������н��е�10minʱǡ�ô�ƽ�⣬��ø���ֵ�Ũ�����£�| ���� | CH3OH | CH3OCH3 | H2O |
Ũ�ȣ�(mol��L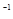 ) ) | 0.44 | 0.6 | 0.6 |
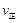 (�״�)������
(�״�)������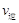 (ˮ)�������������������)��
(ˮ)�������������������)��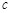 (CH3OH)
(CH3OH)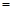 ������������
������������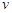 (CH3OH)
(CH3OH)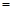 ������������
�������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
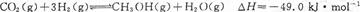
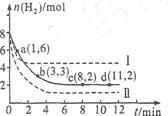
| A��O��1 min | B��1��3 min | C��3��8 min | D��8��11 min |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
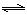 2CO��g����K1
2CO��g����K1 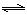 H2��g��+CO2��g����K2
H2��g��+CO2��g����K2 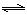 CO��g��+H2��g����K3
CO��g��+H2��g����K3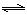 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
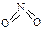 ��
��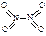 ����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ ��
����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ �� 2NO2(g)�����¶�ΪT1
2NO2(g)�����¶�ΪT1 ��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��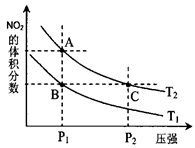
 ��ķ�Ӧ���ʣ�A��C
��ķ�Ӧ���ʣ�A��C �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com