
��16�֣�����ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ�����⡣���պϸ�װ�õĵ��ʱ���۲쵽�����Ƶ�ָ�뷢����ƫת����ش��������⣺
(1)��װ�õ�������____________ (�ԭ��ء����ء�)��
(2) д���缫��Ӧʽ�� Pt��______ _ �� �����в���0��1 mol����ʱ����������ͭ������ӦΪ___________________��
(3)��������Һ���䣬����缫������ͭ�缫������պ�һ��ʱ���������Һ����ɫ___________������������dz�����ޱ仯������
����ȼ�ϵ����ȼ�ϣ���CO��H2��CH4�ȣ���O2�����������Ӧ������ѧ��ת��Ϊ���ܵ�װ�á�������ǿ��Ϊ����ʵ�CH4ȼ�ϵ�أ������缫��ӦʽΪ��
���ŷŵ�Ľ��У���Һ��pH ��������С�����䡱��
������Cu2+��Cl- ��Na+��SO42-���������е�����������ɵĵ������Һ�����֣���ѡ��ͭ�缫�����缫���е��ʵ�顣
(1)Ҫʹ�����������ʵ���ɺ��������䣬����ϡ��Һ��Ũ�����������ǣ�Ӧ��______Ϊ�������________ ��Һ�������缫��ӦʽΪ____________________________ ��
(2)�Բ���������� ________ ��Һʱ����Һ�ļ�����������ǿ������Һ�����壬�����ܷ�ӦʽΪ __________________________ ��
����1��ԭ��� ��1�֣� ��2��2Cl�� - 2e���� Cl2����2�֣� 6.4g ��2�֣�
��3���ޱ仯 ��1�֣�
����CH4+10OH����8e=CO32-+7H2O ��2�֣� ��С��1�֣�
����(1) ����1�֣� Na2SO4 ��1�֣�
4OH-��4e- = 2H2O+O2����2�֣���д2H2O-4e- = O2��+4H+ ͬ�����֣�
(2)
NaCl��1�֣� 2NaCl+2H2O 2NaOH+H2��+Cl2����2�֣�
2NaOH+H2��+Cl2����2�֣�
��ûд����⡱��ͨ�硱�����֣�
������������1����װ����п��ϡ������Է����û���Ӧ�����Լ���ԭ��أ����ǵ��ء�
��2��п��ͭ���ã�����п�Ǹ�����ͭ����������Pt����������Һ�е�������������ʧȥ���ӣ�������������ӦʽΪ2Cl�� - 2e���� Cl2�������в��������������������ʵ�����0.1mol��ת�Ƶ�����0.2mol�����Ը��ݵ��ӵĵ�ʧ�غ��֪����������ͭ�����ʵ�����0.2mol��2��0.1mol��������Ϊ6.4g��
��3��������ͭ�缫��������ͭʧȥ���ӣ�ͬʱ����ͭ�����ֵõ����Ӷ�������������Һ��Ũ���Dz���ģ�����Һ����ɫ�ޱ仯��
�������ڷ�Ӧ��ʧȥ���ӣ������ڸ���ͨ�롣����Ϊ��Һ�Լ��ԣ����Ը����缫��ӦʽΪCH4+10OH����8e��=CO32-+7H2O�������õ����ӣ�������ͨ�룬�缫��ӦʽΪ2O2��8e����4H2O=8OH��,�����ܷ�ӦʽΪCH4��2O2��2OH��=CO32����3H2O��������Һ��pH�Ǽ�С�ġ�
����(1) )Ҫʹ�����������ʵ���ɺ��������䣬�������ˮ�����Ե����������ơ�������OH-�ŵ磬��ӦʽΪ4OH-��4e- = 2H2O+O2����
��2����Һ�ļ�����������ǿ������Һ�����壬���Ե������Ȼ�����Һ����ӦʽΪ
2NaCl+2H2O 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��ԭ�ر�����ѧ��һ�������¿���ѧ�Ծ����������� ���ͣ������
(16��) ijУ��ѧ��ȤС���ͬѧ���������ϵ�֪�����������ܵ�����Ĥ��ʹ��������Χ�Ľ���(������ˮ��)����������ֵ������������Բ�������ʢ�ź������̲ˡ�Ϊ�˸�С���ͬѧ�������������Ĥ������̽������������£������������ա�
(1)������ǯ��סһ���ȥ����Ĥ����Ƭ�����ھƾ��ƻ��������գ���Ƭ����Ӵ�����IJ��ֱ䰵��Ƭ�̺����������ҡ����������ҡ�Σ�ȴ���������������䡣������Ϊ����������Ĥ���۵�________(����ڡ����ڡ�)�ڲ������۵㣬�������ס�����Բ�������������
(2)ȡ����������������סһС������ƺ����ˮ�������������������ЩС���ٰ���סһС������ƺ����ˮ�У�Ѹ�پ��д��������ݲ������Խ������е�ԭ��д����صĻ�ѧ����ʽ��___________ ___________________��
(3)��ɰֽ��ĥһ��Ƭ��ʹ������ֲڣ��ٽ������CuSO4ϡ��Һ�У�2��3 min����������ɫ���帽��������档��д��������ɫ��������ӷ���ʽ�� ��
(4)������δ��ɰֽ��ĥ������Ƭ����������������Һ�У�Ƭ�̺�����������ɫ���塣��д����������Ĥ������������Һ��Ӧ�����ӷ���ʽ��___________________________��
(5)��ȡһ���������ޣ��ռ���һ��CO2�����������Ũ����������Һ�������ѿڷ�ա����Է��������ޡ����ǡ����죬������ˣ���һ����������ֻ����첢���������Խ���Ϊʲô��д���йص����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����һ�и�����ѧ�ڵڶ��ζ�ʱ��ϰ��ѧ�Ծ� ���ͣ�ʵ����
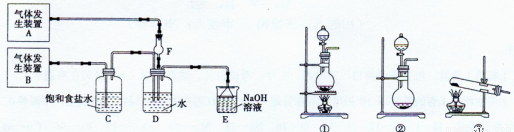
��11�֣�Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ���������ͼ��ʾ��ʵ��װ�á�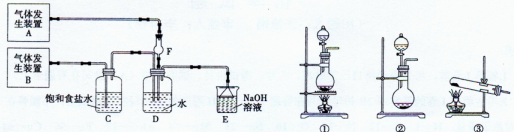
����գ�
��1���û�ѧ��ȤС���ͬѧΪ��ȡCl2��SO2���壬�ֲ���Na2SO3��70%��Ũ����Ϊԭ����ȡSO2������MnO2��Ũ���ᣨ12 mol��L-1��Ϊԭ����ȡCl2���ڴ�ʵ���У�F������������ �����巢��װ��BӦѡ��١��ڡ�������װ���е� ��ѡ����ţ���
��2��Dװ������Ҫ��Ӧ�����ӷ���ʽΪ�� ��
��3��Ϊ��֤ͨ��Dװ����������Cl2��������SO2��������ȤС���ͬ!ѧ���������Լ����Ȼ�����Һ���Ȼ�������Һ�����軯����Һ��������Һ��Ʒ����Һ�����Ը��������Һ��
��Cl2����ȡ����D����Һ�μ���ʢ�� ���Լ����ƣ���ͬ�����Թ��У��ټ�������������� ��˵��Cl2������
��SO2������ȡ����D����Һ�μ���ʢ�� ���Թ��ڣ������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��һ�������¿���ѧ�Ծ��������棩 ���ͣ������
(16��) ijУ��ѧ��ȤС���ͬѧ���������ϵ�֪�����������ܵ�����Ĥ��ʹ��������Χ�Ľ���(������ˮ��)����������ֵ������������Բ�������ʢ�ź������̲ˡ�Ϊ�˸�С���ͬѧ�������������Ĥ������̽������������£������������ա�
(1)������ǯ��סһ���ȥ����Ĥ����Ƭ�����ھƾ��ƻ��������գ���Ƭ����Ӵ�����IJ��ֱ䰵��Ƭ�̺����������ҡ����������ҡ�Σ�ȴ���������������䡣������Ϊ����������Ĥ���۵�________(����ڡ����ڡ�)�ڲ������۵㣬�������ס�����Բ�������������
(2)ȡ����������������סһС������ƺ����ˮ�������������������ЩС���ٰ���סһС������ƺ����ˮ�У�Ѹ�پ��д��������ݲ������Խ������е�ԭ��д����صĻ�ѧ����ʽ��___________ ___________________��
(3)��ɰֽ��ĥһ��Ƭ��ʹ������ֲڣ��ٽ������CuSO4ϡ��Һ�У�2��3 min����������ɫ���帽��������档��д��������ɫ��������ӷ���ʽ�� ��
(4)������δ��ɰֽ��ĥ������Ƭ����������������Һ�У�Ƭ�̺�����������ɫ���塣��д����������Ĥ������������Һ��Ӧ�����ӷ���ʽ��___________________________��
(5)��ȡһ���������ޣ��ռ���һ��CO2�����������Ũ����������Һ�������ѿڷ�ա����Է��������ޡ����ǡ����죬������ˣ���һ����������ֻ����첢���������Խ���Ϊʲô��д���йص����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ�ڵڶ��ζ�ʱ��ϰ��ѧ�Ծ�1 ���ͣ�ʵ����
��11�֣�Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ���������ͼ��ʾ��ʵ��װ�á�
����գ�
��1���û�ѧ��ȤС���ͬѧΪ��ȡCl2��SO2���壬�ֲ���Na2SO3��70%��Ũ����Ϊԭ����ȡSO2������MnO2��Ũ���ᣨ12 mol��L-1��Ϊԭ����ȡCl2���ڴ�ʵ���У�F������������ �����巢��װ��BӦѡ��١��ڡ�������װ���е� ��ѡ����ţ���
��2��Dװ������Ҫ��Ӧ�����ӷ���ʽΪ�� ��
��3��Ϊ��֤ͨ��Dװ����������Cl2��������SO2��������ȤС���ͬ!ѧ���������Լ����Ȼ�����Һ���Ȼ�������Һ�����軯����Һ��������Һ��Ʒ����Һ�����Ը��������Һ��
��Cl2����ȡ����D����Һ�μ���ʢ�� ���Լ����ƣ���ͬ�����Թ��У��ټ�������������� ��˵��Cl2������
��SO2������ȡ����D����Һ�μ���ʢ�� ���Թ��ڣ������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com