
O2ŗĶO3ŹĒŃõŌŖĖŲµÄĮ½ÖÖµ„ÖŹ£¬øł¾ŻĘä·Ö×Ó×é³É»ņŠŌÖŹĶź³ÉĻĀĮŠø÷Ģā£ŗ
£Ø1£©³ōŃõ·¢ÉśĘ÷½«ŃõĘų×Ŗ»ÆĪŖ³ōŃõµÄ·“Ó¦ŹōÓŚ ±ä»Æ£»
£Ø2£©µČĪļÖŹµÄĮæµÄO2ŗĶO3Ėłŗ¬·Ö×ÓøöŹż±ČĪŖ £¬ĖüĆĒµÄÖŹĮæÖ®±ČĪŖ £»
£Ø3£©µČĪĀµČŃ¹ĻĀ£¬µČĢå»żµÄO2ŗĶO3Ėłŗ¬·Ö×ÓøöŹż±ČĪŖ £»
£Ø4£©O3ÓėKIČÜŅŗ·“Ӧɜ³ÉµÄĮ½ÖÖµ„ÖŹ·Ö±šŹĒ ŗĶ £ØĢī·Ö×ÓŹ½£©£»
£Ø5£©ÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬Čē¹ūAgŃõĘųÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖb£¬Ōņc g³ōŃõŌŚ±ź×¼×“æöĻĀµÄĢå»żŌ¼ŹĒ £ØÓĆŗ¬NA”¢a”¢b”¢cµÄŹ½×Ó±ķŹ¾£©”£
£Ø1£©»Æѧ £Ø2£©1£ŗ1, 2£ŗ3 (3) 1£ŗ1 (4) O2”¢I2
£Ø5£©44.8bc/3a.NA»ņ14.9bc/a.NA£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŃõĘųÓė³ōŃõŹĒ²»Ķ¬µÄĪļÖŹ£¬Ö®¼äµÄ×Ŗ»ÆŹōÓŚ»Æѧ±ä»Æ£»
£Ø2£©µČĪļÖŹµÄĮæµÄO2ŗĶO3Ėłŗ¬·Ö×ÓøöŹż±ČŅ²¼“ĪļÖŹµÄĮæÖ®±ČĪŖ1:1£¬ÖŹĮæÖ®±ČŅ²¼“Ħ¶ūÖŹĮæÖ®±ČĪŖ2:3£»
£Ø3£©µČĪĀµČŃ¹ĻĀ£¬µČĢå»żµÄO2ŗĶO3µÄĪļÖŹµÄĮæŅ²ĻąĶ¬£¬ĖłŅŌ·Ö×ÓŹżÖ®±ČŅ²¼“ĪļÖŹµÄĮæÖ®±ČĪŖ1:1£»
£Ø4£©O3ÓėKIČÜŅŗ·“Ӧɜ³ÉµÄĮ½ÖÖµ„ÖŹ“ÓŌŖĖŲÖÖĄą½Ē¶Č²»ÄŃÅŠ¶ĻÖ»ÄÜŹĒO2”¢I2
£Ø5£©AgŃõĘųÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖb£¬Ōņb=a”¤NA/32=" 3a”¤" NA/96, c g³ōŃõŌŚ±ź×¼×“æöĻĀµÄĢå»żV="c”¤22.4/48=" 2c”¤22.4/96=44.8cb/3ANA=14.9bc/a.NA
æ¼µć£ŗÖ÷ŅŖæ¼²éĘųĢåĪļÖŹµÄĮæ”¢ÖŹĮ攢Į£×ÓŹż”¢Ģå»żÖ®¼äµÄ»»Ėć¹ŲĻµ


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©ÓĆ9mol/LµÄÅØĮņĖįĻ”ŹĶ³É 0£®9mol/LµÄĻ”ĮņĖį 100mL £¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÅäÖĘ²Ł×÷æÉ·Ö½ā³ÉČēĻĀ¼ø²½£¬ŅŌĻĀÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ_____________________
A ĻņČŻĮæĘæ֊עČėÉŁĮæÕōĮóĖ®£¬¼ģ²éŹĒ·ńĀ©Ė®
B ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±¼°²£Į§°ō£¬½«ČÜŅŗ×¢ČėČŻĮæĘ棬²¢ÖŲø“²Ł×÷Į½“Ī
C ÓĆŅŃĄäČ“µÄĻ”ĮņĖį×¢ČėŅŃ¼ģ²é²»Ā©Ė®µÄČŻĮæĘæÖŠ
D øł¾Ż¼ĘĖć£¬ÓĆĮæĶ²ĮæČ”Ņ»¶ØĢå»żµÄÅØĮņĖį
E£®½«ÅØĮņĖįŃŲÉÕ±±ŚĀżĀż×¢ČėŹ¢ÓŠÕōĮóĖ®µÄŠ”ÉÕ±ÖŠ£¬²¢²»¶ĻÓĆ²£Į§°ō½Į°č
F£®øĒÉĻČŻĮæĘæČū×Ó£¬Õńµ“£¬Ņ”ŌČ
G£®ÓĆ½ŗĶ·µĪ¹ÜµĪ¼ÓÕōĮóĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻąĒŠ
H£®¼ĢŠųĶłČŻĮæĘæÖŠŠ”ŠÄµŲ¼ÓÕōĮóĖ®£¬Ź¹ŅŗĆę½Ó½üæĢ¶ČĻß1~2 cm
£Ø2£©Čē¹ūŹµŃéŹŅÓĆ98£„µÄÅØĮņĖį(ĆܶČĪŖ1£®8g”¤cm-3 ) ÅäÖĘ3£® 6 mol”¤L-1µÄĻ”ĮņĖį250mL”£¼ĘĖćĖłŠčÅØĮņĖįµÄĢå»żĪŖ_____________mL”£
£Ø3£©ÓÉÓŚ“ķĪó²Ł×÷, Ź¹µĆµ½µÄÅØ¶ČŹż¾Ż±ČÕżČ·µÄĘ«“óµÄŹĒ___________£ØĢīŠ“ŠņŗÅ£©”£
A Ź¹ÓĆČŻĮæĘæÅäÖĘČÜŅŗŹ±, ø©ŹÓŅŗĆę¶ØČŻŗóĖłµĆČÜŅŗµÄÅضČ
B ƻӊÓĆÕōĮóĖ®Ļ“ÉÕ±2-3“Ī£¬²¢½«Ļ“ŅŗŅĘČėČŻĮæĘæÖŠ
C ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»£¬Ć»ÓŠŗęøÉ
D ¶ØČŻŹ±£¬µĪ¼ÓÕōĮóĖ®£¬ĻČŹ¹ŅŗĆęĀŌøßÓŚæĢ¶ČĻߣ¬ŌŁĪü³öÉŁĮæĖ®Ź¹ŅŗĆę°¼ĆęÓėæĢ¶ČĻßĻąĒŠ
E£®°ŃÅäŗƵÄČÜŅŗµ¹ČėÓĆÕōĮóĖ®Ļ“¾»¶ųĪ“øɵďŌ¼ĮĘæÖŠ±øÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øł¾ŻĻĀĆęĪļÖŹ¼ä×Ŗ»ÆµÄæņĶ¼£¬»Ų“šÓŠ¹ŲĪŹĢā£»
`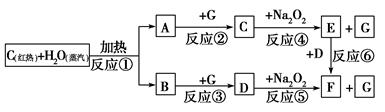
(1)ÓÉ·“Ó¦¢Ł²śÉśµÄA”¢B»ģŗĻĪļµÄ¹¤ŅµĆū³ĘŹĒ________”£
(2)Š“³öæņĶ¼ÖŠD”¢EµÄ»ÆѧŹ½£ŗD________£»E________”£
(3)Čē¹ū2 mol Na2O2Óė×ćĮæĖ®ÕōĘų·“Ó¦£¬æɵƱź×¼×“æöĻĀĘųĢåµÄĢå»żŹĒ________L£¬Ķ¬Ź±·“Ó¦ÖŠ×ŖŅʵē×Ó×ÜŹżŹĒ____________”£(NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż)
(4)Čē¹ūA”¢B»ģŗĻĘųĢå7.8 g£¬ŌŚÓėG³ä·Ö·“Ó¦ŗó£¬Ķعż×ćĮæNa2O2²ć£¬æÉŹ¹Na2O2ŌöÖŲ________g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)ŌŚŅ»¶ØĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬1Ģå»żX2(g)ŗĶ3Ģå»żY2(g)»ÆŗĻÉś³É2Ģå»żZ(g)£¬ŌņZĘųĢåµÄ»ÆѧŹ½ŹĒ________”£
(2)ŌŚĶ¬ĪĀ”¢Ķ¬Ń¹ĻĀ£¬ŹµŃé²āµĆCO”¢N2ŗĶO2ČżÖÖĘųĢåµÄ»ģŗĻĘųĢåµÄĆܶȏĒH2µÄ14.5±¶£¬ĘäÖŠO2µÄÖŹĮæ·ÖŹżĪŖ________”£ČōĘäÖŠCOŗĶN2µÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć1£¬Ōņ»ģŗĻĘųĢåÖŠŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ________”£
(3)ĻąĶ¬Ģõ¼žĻĀ£¬Ä³Cl2ÓėO2»ģŗĻĘųĢå100 mLĒ”ŗĆÓė150 mL H2»ÆŗĻÉś³ÉHClŗĶH2O£¬Ōņ»ģŗĻĘųĢåÖŠCl2ÓėO2µÄĢå»ż±ČĪŖ________£¬»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ________”£
(4)ĻÖÓŠm gijĘųĢ壬ĖüµÄĦ¶ūÖŹĮæĪŖM g”¤mol£1£¬Ōņ
¢ŁøĆĘųĢåČÜÓŚ1 LĖ®ÖŠ(²»æ¼ĀĒ·“Ó¦)£¬ĘäČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ________”£
¢ŚøĆĘųĢåČÜÓŚĖ®ŗóŠĪ³ÉV LČÜŅŗ£¬ĘäČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ______ mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖĮņĖį”¢°±Ė®µÄĆܶČÓėĖł¼ÓĖ®µÄĮæµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻÖÓŠĮņĖįÓė°±Ė®ø÷Ņ»·Ż£¬Ēėøł¾Ż±ķÖŠŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
| | ČÜÖŹµÄĪļÖŹµÄĮæ ÅضČ/mol”¤L£1 | ČÜŅŗµÄĆܶČ/g”¤cm£3 |
| ĮņĖį | c1 | ¦Ń1 |
| °±Ė® | c2 | ¦Ń2 |
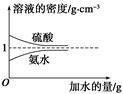
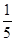 c2 mol”¤L£1µÄ°±Ė®µČÖŹĮæ»ģŗĻ£¬ĖłµĆČÜŅŗµÄĆܶČ________(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£¬ĻĀĶ¬)¦Ń2 g”¤cm£3£¬ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅضČ________
c2 mol”¤L£1µÄ°±Ė®µČÖŹĮæ»ģŗĻ£¬ĖłµĆČÜŅŗµÄĆܶČ________(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£¬ĻĀĶ¬)¦Ń2 g”¤cm£3£¬ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅضČ________ 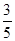 c2 mol”¤L£1(Éč»ģŗĻŗóČÜŅŗµÄĢå»ż±ä»ÆŗöĀŌ²»¼Ę)”£
c2 mol”¤L£1(Éč»ģŗĻŗóČÜŅŗµÄĢå»ż±ä»ÆŗöĀŌ²»¼Ę)”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø¢ń£©ŌŚŹŅĪĀĻĀ£¬ĻņijŅ»ČŻ»ż¹Ģ¶ØµÄÕęæÕČŻĘ÷ÄŚ³äČė¶”Ķé£ØĘų£©ŗĶŃõĘų£¬Ź¹ČŻĘ÷ÄŚ»ģŗĻĘųµÄ×ÜŃ¹Ēæ“ļµ½p1£¬µć»šČ¼ÉÕ£¬ŃõĘų·“Ó¦ĶźČ«£¬ĄäČ“ÖĮŹŅĪĀŗóČŻĘ÷ÄŚĘųĢåµÄ×ÜŃ¹ĒæĪŖp2”£
£Ø1£©Čō¶”ĶéČ¼ÉÕµÄÉś³ÉĪļÖ»ÓŠH2O£ØŅŗ£©ŗĶCO2£¬Ōņp2/p1£½ ”£
£Ø2£©Čō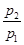 £½0.64£¬Ōņ·“Ó¦Ē°»ģŗĻĘųÖŠ¶”ĶéµÄĪļÖŹµÄĮæ·ÖŹż£½ ”£
£½0.64£¬Ōņ·“Ó¦Ē°»ģŗĻĘųÖŠ¶”ĶéµÄĪļÖŹµÄĮæ·ÖŹż£½ ”£
£Ø¢ņ£©Éč°¢·ü¼ÓµĀĀŽ³£ŹżĪŖNA£¬ŌŚ³£ĪĀ³£Ń¹ĻĀĘųĢåµÄĦ¶ūĢå»żĪŖVm L”¤mol£1£¬O2ŗĶN2µÄ»ģŗĻĘųĢåa gŗ¬ÓŠbøö·Ö×Ó£¬Ōņc gøĆ»ģŗĻĘųĢåŌŚ³£ĪĀ³£Ń¹ĻĀĖłÕ¼µÄĢå»żÓ¦ŹĒ L”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°Ń2.0mol/LCuSO4ČÜŅŗŗĶ1.0mol/LH2SO4ČÜŅŗµČĢå»ż»ģŗĻ£Ø¼ŁÉč»ģŗĻŗóČÜŅŗµÄĢå»żµČÓŚĮ½ČÜŅŗµÄĢå»żÖ®ŗĶ£©”£
£Ø1£©ČÜŅŗÖŠH+µÄĪļÖŹµÄĮæÅضČĪŖ £¬SO42-µÄĪļÖŹµÄĮæÅضČĪŖ ”£
£Ø2£©Ļņ»ģŗĻČÜŅŗÖŠ¼ÓČė×ćĮæµÄĢś·Ū£¬¾×ć¹»³¤µÄŹ±¼äŗó£¬Ģś·ŪÓŠŹ£Óą”£“ĖŹ±£¬ČÜŅŗÖŠµÄFe2+ĪļÖŹµÄĮæÅضČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³Ń§ÉśÓūÅäÖĘ6£®0 mol·L£1µÄH2SO4 1 000 mL£¬ŹµŃéŹŅÓŠČżÖÖ²»Ķ¬ÅØ¶ČµÄĮņĖį£ŗ¢Ł480 mL 0£®5 mol·L£1µÄĮņĖį£»¢Ś150 mL 25%µÄĮņĖį(¦Ń£½1£®18 g·mL£1)£»¢Ū×ćĮæµÄ18 mol·L£1µÄĮņĖį”£ÓŠČżÖÖ¹ęøńµÄČŻĮæĘæ£ŗ250 mL”¢500 mL”¢1 000 mL”£ĄĻŹ¦ŅŖĒó°Ń¢Ł¢ŚĮ½ÖÖĮņĖįČ«²æÓĆĶź£¬²»×ćµÄ²æ·ÖÓÉ¢ŪĄ“²¹³ä”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃéĖłÓĆ25%µÄĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ________mol·L£1(±£Įō1Ī»Š”Źż)”£
£Ø2£©ÅäÖĘøĆĮņĖįČÜŅŗӦєÓĆČŻĮæĘæµÄ¹ęøńĪŖ________mL”£
£Ø3£©ÅäÖĘŹ±£¬øĆĶ¬Ń§µÄ²Ł×÷Ė³ŠņČēĻĀ£¬Ēė½«²Ł×÷²½ÖčB”¢D²¹³äĶźÕū”£
A£®½«¢Ł¢ŚĮ½ČÜŅŗČ«²æŌŚÉÕ±ÖŠ»ģŗĻ¾łŌČ£»
B£®ÓĆĮæĶ²×¼Č·ĮæČ”ĖłŠčµÄ 18 mol·L£1µÄÅØĮņĖį mL£¬ŃŲ²£Į§°ōµ¹ČėÉĻŹö»ģŗĻŅŗÖŠ”£
²¢ÓĆ²£Į§°ō½Į°č£¬Ź¹Ęä»ģŗĻ¾łŌČ£»
C£®½«»ģŗĻ¾łŌȵÄĮņĖįŃŲ²£Į§°ō×¢ČėĖłŃ”µÄČŻĮæĘæÖŠ£»
D£®______________________________________£»
E£®Õńµ“£¬¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶ČĻß1”«2 cm“¦£»
F£®øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗµÄ°¼ŅŗĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ£»
G£®½«ČŻĮæĘæøĒ½ō£¬Õńµ“£¬Ņ”ŌČ”£
£Ø4£©Čē¹ūŹ”ĀŌ²Ł×÷D£¬¶ŌĖłÅäČÜŅŗÅضČÓŠŗĪÓ°Ļģ£æ________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
£Ø5£©½ųŠŠ²Ł×÷CĒ°»¹Šč×¢Ņā________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°±ŗĶĮŖ°±(N2H4)ŹĒµŖµÄĮ½ÖÖ³£¼ū»ÆŗĻĪļ£¬ŌŚæĘѧ¼¼ŹõŗĶÉś²śÖŠÓŠÖŲŅŖµÄÓ¦ÓĆ”£øł¾ŻĢāŅāĶź³ÉĻĀĮŠ¼ĘĖć£ŗ
(1)ĮŖ°±ÓĆŃĒĻõĖįŃõ»ÆÉś³ÉµŖµÄĮķŅ»ÖÖĒā»ÆĪļ£¬øĆĒā»ÆĪļµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ43.0£¬ĘäÖŠµŖŌ×ÓµÄÖŹĮæ·ÖŹżĪŖ0.977£¬¼ĘĖćČ·¶ØøĆĒā»ÆĪļµÄ·Ö×ÓŹ½ĪŖ________”£øĆĒā»ÆĪļŹÜײ»÷ŌņĶźČ«·Ö½āĪŖµŖĘųŗĶĒāĘų”£4.30 gøĆĒā»ÆĪļŹÜײ»÷ŗó²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________L”£
(2)ĮŖ°±ŗĶĖÄŃõ»Æ¶žµŖæÉÓĆ×÷»š¼żĶĘ½ų¼Į£¬ĮŖ°±ŹĒČ¼ĮĻ£¬ĖÄŃõ»Æ¶žµŖ×öŃõ»Æ¼Į£¬·“Ó¦²śĪļŹĒµŖĘųŗĶĖ®”£ÓÉĮŖ°±ŗĶĖÄŃõ»Æ¶žµŖ×é³ÉµÄ»š¼żĶĘ½ų¼ĮĶźČ«·“Ӧɜ³É72.0 kgĖ®£¬ŌņĶĘ½ų¼ĮÖŠĮŖ°±µÄÖŹĮæ________”£
(3)°±µÄĖ®ČÜŅŗæÉÓĆÓŚĪüŹÕNOÓėNO2»ģŗĻĘųĢ壬·“Ó¦·½³ĢŹ½ĪŖ6NO£« 4NH3=5N2£«6H2O ””6NO2£« 8NH3=7N2£«12H2O”£NOÓėNO2»ģŗĻĘųĢå180 mol±»8.90”Į103g°±Ė®(ÖŹĮæ·ÖŹż0.300)ĶźČ«ĪüŹÕ£¬²śÉś156 molµŖĘų”£ĪüŹÕŗó°±Ė®ĆܶČĪŖ0.980 g/cm3”£Ōņ¢ŁøĆ»ģŗĻĘųĢåÖŠNOÓėNO2µÄĢå»ż±ČĪŖ________£¬¢ŚĪüŹÕŗó°±Ė®µÄĪļÖŹµÄĮæÅضČ________(“š°ø±£Įō1Ī»Š”Źż)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com