

���� ��֪ij���������к���NaCl���ʣ��ж��ַ����ⶨ�����д��������������
��I�����巨
�ٰ�ͼ��װ�����������װ�õ������ԣ�
�ڽ�ag����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�۴�A�����ܻ�������һ�����Ŀ������Ͼ�װ���ڵĿ�������������гɷָ��ţ�
�ܳ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�b��
�ݴӷ�Һ©������6mol•L-1�����ᣬֱ�����ٲ�������ʱΪֹ��
���ٴ�A�����ܻ�������һ�����Ŀ����������ɵĶ�����̼����ȫ���ϵ�E������
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c��
���ظ�����͢ߵIJ�����ֱ��U�ܵ������������䣬Ϊd�ˣ��ݴ˼������ɶ�����̼��������
��1��װ��C����ˮ���ն�����̼�����е��Ȼ��⣬װ��D����Ũ���ᣬ���ն�����̼�е�ˮ������
��2��װ��F��Ϊ�˷�ֹ������ˮ�����Ͷ�����̼����װ��Eʹ�ⶨ���������
��3�����ɶ�����̼����=��d-b��g�����̼Ԫ���غ����̼���Ƶ�����������
��II��������
�ڸ�ʵ��ϴ�Ӳ����м��������ϴ�ɾ��ķ�����ȡ���һ��ϴ��Һ������ϡHNO3�ữ��Ȼ����뼸��AgNO3��Һ���������ӵĴ����ж��Ƿ�ϴ�Ӹɾ���
�����
��ȡ25.00ml��Һ����ƿ�У����뼸�η�̪��ָʾ������0.1000mol/L��������Һ���еζ��������յ�ʱ������20.00ml���ᣬ�����ķ�ӦΪ��Na2CO3+HCl=NaHCO3+NaCl�����ݻ�ѧ��Ӧ�Ķ�����ϵ����̼���Ƶ����ʵ����õ���Ʒ�е�̼���Ƶ�����������õ�̼���Ƶ�����������
���ݵζ������б���Һ����ı仯�����жϲ�������c�����⣩=$\frac{c������V������}{V�����⣩}$��
A��������Ʒ��Һ����ʱ��������ƿ�̶��ߣ�����ˮ������δ�ﵽ�̶��ߣ�
B��������Ʒ��Һ���ݺ�ҡ�ȣ�����Һ���������ƿ�̶��ߣ������ּ�ˮ���̶��ߣ�������Һ�����̶��ߣ�Ũ�ȼ�С��
C��������ζ�ʱ�ζ�ǰ���ӿ̶��߶�����ȡ�������ζ��յ�ʱ���ӿ̶��߶�������ȡ��ֵ��С��
D���ζ�ǰ��ƿ����ˮ�Բⶨ�����Ӱ�죻
E����ʽ�ζ��ܵζ�ǰ�����ݣ����ζ���������ʧ������Һ������ⶨ�������
��� �⣺��I�����巨
�ٰ�ͼ��װ�����������װ�õ������ԣ�
�ڽ�ag����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�۴�A�����ܻ�������һ�����Ŀ������Ͼ�װ���ڵĿ�������������гɷָ��ţ�
�ܳ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�b��
�ݴӷ�Һ©������6mol•L-1�����ᣬֱ�����ٲ�������ʱΪֹ��
���ٴ�A�����ܻ�������һ�����Ŀ����������ɵĶ�����̼����ȫ���ϵ�E������
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c��
���ظ�����͢ߵIJ�����ֱ��U�ܵ������������䣬Ϊd�ˣ��ݴ˼������ɶ�����̼��������
��1��װ��C����ˮ���ն�����̼�����е��Ȼ��⣬װ��D����Ũ���ᣬ���ն�����̼�е�ˮ������C��D��װ���зֱ�װ���Լ�Ϊ��ˮ��Ũ���ᣬ
�ʴ�Ϊ��ˮ��Ũ���
��2��װ��F��Ϊ�˷�ֹ������ˮ�����Ͷ�����̼����װ��Eʹ�ⶨ���������
�ʴ�Ϊ����ֹ�����е�CO2��ˮ������U���У�
��3�����ɶ�����̼����=��d-b��g�����̼Ԫ���غ����̼���Ƶ���������=$\frac{\frac{��d-b��g}{44g/mol}��106g/mol}{ag}$��100%=$\frac{106��d-b��}{44a}$��100%��
�ʴ�Ϊ��$\frac{106��d-b��}{44a}$��100%��
��II���ڸ�ʵ��ϴ�Ӳ����м��������ϴ�ɾ��ķ�����ȡ���һ��ϴ��Һ������ϡHNO3�ữ��Ȼ����뼸��AgNO3��Һ���������ӵĴ����ж��Ƿ�ϴ�Ӹɾ���ʵ�����Ϊ��ȡ����ϴ��Һ���Թ��м���ϡHNO3�ữ��Ȼ����뼸��AgNO3��Һ����û�а�ɫ����֤����ϴ�ɾ�������û��ϴ�ɾ���
�ʴ�Ϊ��ȡ����ϴ��Һ���Թ��м���ϡHNO3�ữ��Ȼ����뼸��AgNO3��Һ����û�а�ɫ����֤����ϴ�ɾ�������û��ϴ�ɾ���
��III�����
��5����ȡ25.00ml��Һ����ƿ�У����뼸�η�̪��ָʾ������0.1000mol/L��������Һ���еζ��������յ�ʱ������20.00ml���ᣬ�����ķ�ӦΪ��Na2CO3+HCl=NaHCO3+NaCl�����ݻ�ѧ��Ӧ�Ķ�����ϵ����̼���Ƶ����ʵ����õ���Ʒ�е�̼���Ƶ�������
Na2CO3+HCl=NaHCO3+NaCl��
1 1
n 0.1000mol/L��0.0200L
n=0.0020mol
250ml��Һ�к�̼�������ʵ���=0.0020mol��$\frac{250}{25}$=0.0200mol
̼���Ƶ���������=$\frac{0.0200mol��106g/mol}{2.6500g}$��100%=80.0%��
����c�����⣩=$\frac{c������V������}{V�����⣩}$�����жϣ�
A��������Ʒ��Һ����ʱ��������ƿ�̶��ߣ�����ˮ������δ�ﵽ�̶��ߣ���ҺŨ������A�����ϣ�
B��������Ʒ��Һ���ݺ�ҡ�ȣ�����Һ���������ƿ�̶��ߣ������ּ�ˮ���̶��ߣ�������Һ�����̶��ߣ�Ũ�ȼ�С����B���ϣ�
C��������ζ�ʱ�ζ�ǰ���ӿ̶��߶�����ȡ�������ζ��յ�ʱ���ӿ̶��߶�������ȡ��ֵ��С���õ�����Һ���������С���ⶨŨ�ȼ�С����C���ϣ�
D���ζ�ǰ��ƿ����ˮ�Բⶨ�����Ӱ�죬��D�����ϣ�
E����ʽ�ζ��ܵζ�ǰ�����ݣ����ζ���������ʧ������Һ�����������c�����⣩=$\frac{c������V������}{V�����⣩}$�����жϲⶨ�������E�����ϣ�
�ʴ�Ϊ��80.0%��B��C��
���� ���⿼��ѧ����ʵ��ԭ����װ�����⡢�Բ��������ۡ����ʺ����IJⶨ���к͵ζ�����ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���������֪ʶ���������������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٣��ڣ��ۣ��� | B�� | �ڣ��٣��ܣ��� | C�� | �ڣ��٣��ۣ��� | D�� | �ܣ��ۣ��٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4���ӵı���ģ�ͣ� | B�� | NH4Cl�ĵ���ʽ��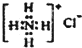 | ||
| C�� | S2-�ṹʾ��ͼ�� | D�� | �۱�ϩ�Ľṹ��ʽΪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ҹ���ѧ�����������ֲ��ɹ���ȡ�������أ�һ������ű����ҩ������2015��ŵ��������ѧ��ҽѧ���������ؽṹ��ʽ��ͼ���ṹ���й���������H2O2�����ƵĻ�ѧ���ʣ������й��������ص�˵������ȷ���ǣ�������
�ҹ���ѧ�����������ֲ��ɹ���ȡ�������أ�һ������ű����ҩ������2015��ŵ��������ѧ��ҽѧ���������ؽṹ��ʽ��ͼ���ṹ���й���������H2O2�����ƵĻ�ѧ���ʣ������й��������ص�˵������ȷ���ǣ�������| A�� | �����л��� | |
| B�� | �����ؾ���һ���������� | |
| C�� | �����ػ�ѧʽΪC15H20O5 | |
| D�� | ��������һ��������������NaOH��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����������ϡ���ױƷ��ҽҩ�����Ϻй���֬�ȵ���Ҫԭ�ϣ�ʵ���������з�Ӧ��ȡ����ᣮ
����������ϡ���ױƷ��ҽҩ�����Ϻй���֬�ȵ���Ҫԭ�ϣ�ʵ���������з�Ӧ��ȡ����ᣮ
| ����ȩ | ������ | ����� | ���� | |
| �ܽ�ȣ�25�棬g/100gˮ�� | 0.3 | ����ˮˮ�� | 0.04 | ���� |
| �е㣨�棩 | 179.6 | 138.6 | 300 | 118 |
| ��Է������� | 106 | 102 | 148 | 60 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com