
X��Y��Z��W��Ϊ10���ӵķ��ӻ����ӡ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣����֮��ת����ϵ��ͼ��ʾ����ش�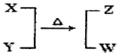
��1����ҵ��ÿ��ȡ1molZҪ�ų�46.2 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ҵ��ȡZ�Ļ�ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 | ���� |
| K/(mol��L��1)��2 | 4.1��106 | K1 | K2 | ���� |

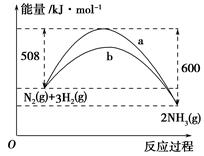
��1��N2(g)+3H2 2NH3(g) ��H=��92.4KJ/mol��3�֣�
2NH3(g) ��H=��92.4KJ/mol��3�֣�
(2) �� > ��CD
��3����4NH+5O2 4NO+6H2O ������ˮ�Ͷ���İ���
4NO+6H2O ������ˮ�Ͷ���İ���
(4)3 NO2+H2O=2HNO3+NO��4NO2+O2+2H2O=4HNO3
(5)����NH3��NO
���������������1��5��ԭ�Ӻ˵�10���ӵ���һ��ΪCH4��NH4+NH4+��WΪ��ɫҺ��,10���ӵ���ɫҺ��ΪH2O�����Բ���÷�ӦΪNH4+��OH-��ȡNH3�ķ�Ӧ��XΪNH4+��YΪOH-��ZΪNH3����ҵ��ȡ�����Ļ�ѧ��ӦΪN2+3H2 2NH3��ÿ��ȡ1molNH3Ҫ�ų�46.2kJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2
2NH3��ÿ��ȡ1molNH3Ҫ�ų�46.2kJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2 2NH3(g) ��H=��92.4KJ/mol
2NH3(g) ��H=��92.4KJ/mol
��2������ȡ�����ķ�ӦΪ���ȷ�Ӧ�������¶�������ƽ�������ƶ���ƽ�ⳣ����С������K1>K2
��A����ƽ��ʱ�����ڸ����ʵ�Ũ��֮�Ȳ�һ��Ϊ��ѧ�������ȣ�����B�����ݵ������У�������ܶ�ʼ�ղ��䣬����C�����ŷ�Ӧ�Ľ��У������ѹǿ��С����ƽ��ʱ���ٱ仯����ȷ��D�����ŷ�Ӧ�Ľ��У���������ʵ�����С���������Է�������������ƽ��ʱ���ٱ仯����ȷ����ѡCD��
��3����A�з�����Ӧ�ǰ����Ĵ��������仯ѧ����ʽ��4NH+5O2 4NO+6H2O
4NO+6H2O
��ŨH2SO4������ˮ�ԣ�Ҳ���ԺͰ�����Ӧ������B��ŨH2SO4������������ˮ�Ͷ���İ���
��4�����ڿ����IJ��Ϲ��룬����������ˮ��������ȫ��Ӧ�������ᣬ��ѧ����ʽΪ4NO2+O2+2H2O=4HNO3��3 NO2+H2O=2HNO3+NO
��5��a��ͨ����������þ��Dz�������NH3��NO
���㣺����10���������ƶϣ������ĺϳɣ��������ȡ����ѧƽ�ⳣ���ıȽϣ�ƽ��״̬���жϣ���ѧ����ʽ����д



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״������������ͼ��ʾ���Իش��������⣺
��1���úϳ�·�߶��ڻ��������ļ�ֵ���� ��
��2��15��20%���Ҵ�����HOCH2CH2NH2��ˮ��Һ���������ԣ������ϳ���·������CO2���ռ��������ӷ���ʽ��ʾ�Ҵ���ˮ��Һ�������Ե�ԭ�� ��
��3��CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5 kJ/mol����H����285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��
2CO2(g) + 6H2(g) CH3OCH3(g) + 3H2O(g)
CH3OCH3(g) + 3H2O(g)
��֪һ��ѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ��±���
| Ͷ�ϱ�[n(H2) / n(CO2)] | 500 K | 600 K | 700 K | 800 K |
| 1.5 | 45% | 33% | 20% | 12% |
| 2.0 | 60% | 43% | 28% | 15% |
| 3.0 | 83% | 62% | 37% | 22% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ƻ�1 mol N��N����H��H����N��H���ֱ���Ҫ���յ�����Ϊ946 kJ��436 kJ��391 kJ������1 mol N2(g)��3 mol H2(g)��ȫת��ΪNH3(g)�������仯����ֵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�ٸ�������ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ____________________________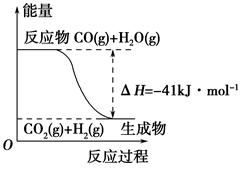
�ڸ�����ͼ��ʾ������ж�����˵������ȷ����______________��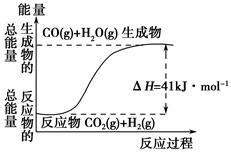
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)=CO2(g)��H2(g)����H��41 kJ��mol��1 |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ԴΣ����ʹ�������Ѱ���µ������Դ�Ϳ�չ��������Դ����Ч���á��ļ����з������ȼ�ϵ��ʹ��������ֱ�Ӳ��������DZ����������ս�ԵĿ���֮һ���Զ��飨��֪�����ȼ����Ϊ2 877.6 kJ��mol��1��Ϊ�����ش��������⣺
��1��д������ȼ�յ��Ȼ�ѧ����ʽ��________________��
��2���������ȼ���ȣ�2 878 kJ��mol��1���춡���ȼ���ȣ�2 869 kJ��mol����������ת��Ϊ�춡��Ĺ�����________����ų��������ա���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ�����Թ�С�ĵط���ʢ��(20 ��)̼�����ϵ��ձ��У��Թ��п�ʼ������������CuSO4��Һ�����õιܵμ�5 mLŨ�������Թ��У��Իش��������⣺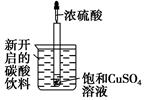
(1)ʵ���й۲쵽��������________________��
(2)�������������ԭ����________________��
(3)�Թ����ƻ���ѧ����������______________���γɵĻ�ѧ��������______________��
(4)д���й�����Ļ�ѧ����ʽ______________��
(5)��ʵ����֪����Ӧ������Һ��������__________(����ڡ�����С�ڡ����ڡ�)Ũ����ͱ���CuSO4��Һ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ķ�������Ϣ���ش����⣺
��1����֪NO2��N2O4�Ľṹʽ�ֱ�Ϊ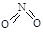 ��
��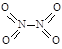 �� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
�� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
��2��ij�ָ��ܳ����ʹ������H2��Ĵ���Ͻ���MH��ʾ������ظ������ϣ�NiO(OH)���������ϣ�KOH��ҺΪ�������Һ�������ĵ缫��ӦΪ��MH��OH����e��= M��H2O����س�ŵ�ʱ���ܷ�ӦΪ��Ni(OH)2��M NiO(OH)��MH
NiO(OH)��MH
�� ��طŵ�ʱ�������ĵ缫��ӦʽΪ ��
�� ������ʱNi(OH)2ȫ��ת��ΪNiO(OH)����������罫��һ���缫����O2��ͬʱ��ɢ����һ���缫�����缫��Ӧ�����ģ���ʱ�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮
��1�����������У�SO2����������SO3��2SO2��g��+O2��g�� 2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺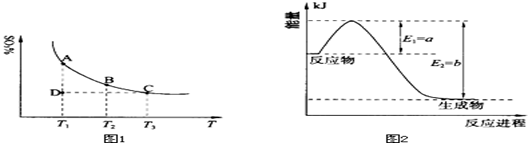
�ٺ��¡���ѹ�����£���Ӧ2SO2��g��+O2��g�� 2SO3��g����ƽ�⣬����ϵ��ͨ�뺤����ƽ�� �ƶ�������������ҡ���������
2SO3��g����ƽ�⣬����ϵ��ͨ�뺤����ƽ�� �ƶ�������������ҡ���������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2�����������������=������ͬ��������Ӧ���е�״̬Dʱ��v�� v�������������������=������ͬ����
��2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
����ͼ2��һ�����¶Ⱥ�ѹǿ����N2��H2��Ӧ����1molNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ��Ӧ����ʽ�� ��
����H����ֵ�ú���ĸa��b�Ĵ���ʽ��ʾ��
�ڰ�������ˮ�õ���ˮ����25���£���a mol?L-1�İ�ˮ��b mol?L-1������������ϣ���Ӧ����Һǡ�������ԣ��ú�a��b�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ������ʽ ��
��3����֪25��CʱKsp[AgCl]=1.6��10-10mol2?L-2��Ksp[AgI]=1.5��10-16mol2?L-2������25���£���0.1L0.002mol?L-1��NaCl��Һ����μ���0.1L0.002mol?L-1��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�У���������0.1L0.002mol?L-1��NaI ��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʹ�������Դ����չ����̼���á�����Ϊ��ѧ���о�����Ҫ���⡣�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�
��1������ˮ����ת������H2����Ҫת����Ӧ���£�
CH4(g) + H2O(g) CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1
CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1
CH4(g) + 2H2O(g) CO2(g) + 4H2(g) ��H=+165��0 kJ��mol��1
CO2(g) + 4H2(g) ��H=+165��0 kJ��mol��1
������Ӧ����ԭ�����е�CO��ʹ�ϳɰ��Ĵ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ���Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4(g) + H2O(g)  CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0
��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����A��B��C���㴦��Ӧƽ�ⳣ����KA��KB��KC���Ĵ�С��ϵΪ___________��(�<������>������="��" )��
��100��ʱ����1 mol CH4��2 mol H2Oͨ���ݻ�Ϊ1 L�Ķ����ܷ������У�������Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����__________
a�������������ܶȺ㶨
b����λʱ��������0��1 mol CH4ͬʱ����0��3 mol H2
c��������ѹǿ�㶨
d��3v��(CH4) = v��(H2)
��3��25��ʱ����20mL0��1mol/L������м���VmL0��1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��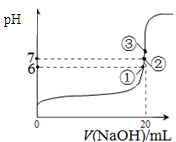
A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9��9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(Na+)��0��1mol/L
��4������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com